Effects of Cannabis Oil on Cannabinoid-Induced Tetrad, Blood Pressure, and Metabolic Parameters in an Experimental Model of Metabolic Syndrome
A B S T R A C T
Objective: The aim of the present study was to analyse the effects of cannabis oil on cannabinoid-induced tetrad, blood pressure, and metabolic parameters in rats normal fed a sucrose-rich diet (SRD).
Methods: Male Wistar rats were fed the following diets for 21 days: Reference Diet (RD): standard commercial laboratory diet, SRD and SRD+Cannabis oil (SRD+Ca): the oral administration of 1 mg/kg of body weight of cannabis oil daily. Cannabis oil present a total cannabinoids CBD:THC 2:1 ratio. During the experimental period, body weight, food intake, blood pressure, body temperature, locomotion, catalepsy and analgesia were evaluated. At the end of the experimental period, levels of glucose, triglycerides, cholesterol, uric acid, AST, ALT and AP in serum were evaluated. In liver were determined AST, ALT and AP enzymes and triglycerides and cholesterol content.
Results: A cannabis oil administration significantly increased (P<0.05) analgesia, and locomotion was also significantly decreased, which was found to be increased in the SRD group. Systolic and diastolic blood pressure decreased during the experiment. In the SRD+Ca group, serum triglycerides and cholesterol levels significantly decreased, reaching values similar to RD group, without changes in glucose levels. In addition, serum uric acid and AP levels significantly decreased, although did not obtain reference values. AST and ALT levels in serum and liver significantly decreased, reaching reference values. At the liver, the triglycerides and cholesterol content significantly decreased with the administration of cannabis oil, although not reaching to reference values.
Conclusions: Our results suggest that cannabis oil could be useful as a therapeutic strategy to prevent some of the alterations present in Metabolic Syndrome, including hypertension, dyslipidemia and liver damage. In addition, the analgesic effect of cannabis oil could be observed in SRD-fed rats.
Keywords
Cannabis oil, cannabinoid-induced tetrad, blood pressure, metabolic parameters, sucrose-rich diet
Graphical Abstract
Introduction
Metabolic Syndrome (MS) increases the risk of Type 2 diabetes and cardiovascular disease. Its main components are dyslipidemia, elevation of arterial blood pressure, dysregulated glucose homeostasis, abdominal obesity and insulin resistance. Recently, other abnormalities such as chronic proinflammatory and prothrombotic states and non-alcoholic fatty liver disease (NASH) have been added to the entity of this Syndrome. MS is a combination of metabolic disorders influenced by genetic and environmental factors, now reaches epidemic proportions in the world [1, 2]. Several studies have experimentally demonstrated that rats fed sucrose-fructose rich diets display many of the metabolic changes observed in the human MS phenotype [3-5]. We previously showed that rats fed a SRD during 3 weeks have dyslipidemia, with a combination of increased VLDL-Tg secretion and a defective removal mechanism of Tg from the circulation. This was accompanied by a significant increase in liver triglyceride storage [6, 7].
Currently, there are only a few specific pharmaceutical strategies available to treat MS. In recent years, the therapeutic properties of the chemical compounds present in Cannabis (cannabinoids) have generated great expectations in society. Although the Cannabis plant has been used in medicine for thousands of years, its therapeutic use currently raises a debate rarely seen in the study of other natural products. Cannabis sativa is native to Asia, it is an annual plant that belongs to the Cannabaceae family. It has been used as a herbal medicine for tens of centuries to treat various diseases and symptoms. Therefore, it is believed that its consumption has positive effects against diseases such as depression, diabetes, multiple sclerosis, glaucoma, asthma, autoimmune diseases and cancer; in pain management, insomnia, lack of appetite, nausea, vomiting, epilepsy and cerebral ischaemia, among others. The most common form of consumption is inhaled or in the form of oil. Adverse reactions are minimal, transitory and of low toxicity, and its use as a complementary and/or alternative treatment to traditional medicine for the aforementioned diseases has been proven [8, 9].
In total, more than a thousand compounds have been identified in the plant, including cannabinoids, terpenes, terpenoids, flavonoids, flavonoid glycosides, polyphenols, steroids, among others. Cannabinoids are classified as phytocannabinoids (of plant origin) to distinguish them from endocannabinoids (non-plant). The main cannabinoids in cannabis include tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabichromene (CBC), their precursor cannabigerol (CBG) and cannabinol (CBN) [10, 11]. Recent studies show that the endocannabinoid system can have a significant influence by decreasing the production of reactive oxygen species, inflammation and subsequent tissue damage, in addition to having some effects on lipid and glucose metabolism. Some recent studies have focused on two cannabinoids, such as CBD and THC, which have great therapeutic potential in inflammatory diseases, diabetes and diabetic complications [12-14].
In this study, we investigate the effects of cannabis oil on cannabinoid-induced tetrad, blood pressure, and metabolic parameters present in experimental Metabolic Syndrome induced by SRD-fed rats. To our knowledge, this is the first study to examine the metabolic effects of oral administration of cannabis oil (CBD:THC 2:1 ratio) in SRD-fed rats.
Materials and Methods
I Cannabis oil Preparation and Characterization
Cannabis oil was obtained from dried inflorescences of the Cannabis sativa CAT1 variety grown at Environmental Research Center (CIM-CONICET-UNLP) (RESOL-2021-3236-APN-MS). Briefly, in order to obtain neutral cannabinoides the inflorescences were first decarboxilated in oven (145 ºC) during 7 min. After that, an alcoholic extraction (10 ml ethanol 96º per gram of inflorescence) was carried out and subsequently the ethanol was evaporated at low temperature with rotavapor (Buchi R 3000). The resulting resin was diluted in corn oil and the cannabinoids in the oil were quantified by HPLC/UV-DAD techniques and analytical standards (Cerilliant Co.) as described in Sedan et al. [15]. Cannabis oil contains 0.60 mg/ml CBD and 0.43 mg/ml THC, with a CBD:THC ratio of 2:1. Finally, the adequate dilution was carried out to obtain the working oil with a concentration of 1 mg/ml.
II Animals and Diets
Male Wistar rats (n=18) purchased from the Veterinary Sciences Institute of Litoral (Instituto de Ciencias Veterinarias del Litoral, ICIVET-Litoral) -Facultad de Veterinaria, Universidad Nacional del Litoral (Esperanza, Santa Fe, Argentina) were maintained with unrestricted access to water and food under controlled temperature (22 ± 1 °C), humidity (50-60%) and air flow conditions, with a fixed 12-h light/dark cycle (light on 07.00 AM to 7.00 PM). Adequate measures were taken to minimize the pain or discomfort of the rats and we used the smallest number of animals possible. This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals and was approved by the Institutional Ethics Committee of the Faculty of Biochemistry and Biological Sciences.
The animals were initially fed a standard powdered rodent commercial diet (GEPSA FEED, Buenos Aires, Argentina). When the rat’s weight was 180-190 g, they were randomly divided into three experimental groups during 21 days: 1) Rats fed a standard powdered rodent commercial diet (Reference diet, RD, n = 6). 2) Rats fed a semisynthetic sucrose rich diet (SRD, n = 6). 3) Rats fed a SRD plus oral administered cannabis oil (SRD+Ca, n = 6). The cannabis oil was administered orally, using a dose of 1 mg/kg body weight daily during the experimental protocol (21 days). The diet compositions are given in (Table 1). The individual body weight and food intake of animals in each group were assessed twice a week throughout the experimental period (21 days). At the end, the food was removed at 07.00 h and experiments were performed between 07.00 and 09.00 h. The animals were anaesthetized with intraperitoneal sodium pentobarbital (60 mg/kg body weight). Blood samples were collected from the inferior vena cava, rapidly centrifuged and serum was either immediately assayed or stored at -20 °C until used. The liver of each rat was totally removed, weighed, and stored at the temperature of liquid N2. The animals were euthanized by removal of vital organ (heart).
Table 1: Composition of experimental diets.
|
SRDa |
|
|
Diet ingredients (g/kg diet) |
|
|
Sucrose |
580 |
|
Casein (vitamin free)b |
165 |
|
Corn oil |
105 |
|
Salt mix |
35 |
|
Vitamin mix |
10 |
|
Choline Chloride |
2 |
|
Methionine |
3 |
|
Fiber (cellulose) |
100 |
|
Energy (kJ/g) |
16.3 |
a The home-made SRD
experimental diet is based on the modified AIN-93M diet. b Casein was
provided by Saputo Molfino Hermanos S.A., Buenos Aires, Argentine.
Reference
diet (RD):
Rodent commercial diet (GEPSA FEED, Buenos Aires, Argentina) containing
(g/kg diet): carbohydrate (corn, sorghum, wheat, oats, barley) 427; protein
180; fat 39; fiber 55; minerals and vitamins 169; moisture 130; digestible
energy 12.5 kJ/g.
III Determination of Blood Pressure
Blood pressure was measured in the three dietary groups in conscious animals during the experimental period using a CODA™ Monitor of tail-cuff non-invasive blood pressure system (Kent Scientific Corporation, Torrington, CT, USA) as previously described [16].
IV Cannabinoid-Induced Tetrad Test
Rats were evaluated for hypolocomotion (open feld test), hypothermia (body temperature), cataleptic (bar test) and analgesic (hot plate test) efects, using the procedures of the tetrad tests as reported by Metna-Laurent et al., with some modifications [17]. Briefly, locomotor activity was assessed 30 min after treatment, for a 5 min period in a polycarbonate box (60 × 60 × 25 cm), and Anymaze software (Stoelting, Wood Dale, Illinois) was used to determine distance traveled. Rectal temperature was assessed 50 min after cannabis oil administration, using an infrared thermometer. Catalepsy was assessed 55 min after cannabis oil administration in a box with a 0.7 cm in diameter placed 4.5 cm off of the ground. The front paws of the rat are placed gently on the horizontal bar and the hind legs on the floor of the cage. The timer is started, stopped when the rat descends from the bar. Analgesia was assessed 85 min after cannabis oil administration in the tail immersion assay, where each rat was hand-held and 1 cm of the tail was submerged into a 52-53°C water bath. The latency for the rat to withdrawal its tail was scored.
V Analytical Methods
Serum triglyceride, cholesterol, uric acid and glucose levels were measured by spectrophotometric methods using commercial enzymatic kits according to the manufacturer’s protocols (Wiener Lab., Argentina; Randox Laboratories Limited, United Kingdom). The activities of serum and hepatic aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (AP) enzymes were measured by spectrophotometric methods using commercial enzymatic kits according to the manufacturer’s protocols (Wiener Lab., Argentina). For preparing the liver homogenate, the frozen tissue was homogenized in 10-volumes of ice-cold 0.1 M potassium phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 rpm for 15 min, and the supernatant used by measure the hepatic damage enzyme activity. Triglycerides and total cholesterol content in liver were extracted with chloroform-methanol (2:1) mixture. Aliquots were evaporated and total cholesterol and triglycerides were analysed using enzymatic methods mentioned before.
VI Statistical Analysis
Results were expressed as mean ± SEM. Statistical comparisons were made transversely between different dietary groups. Data were tested for variance using Levene’s test and normality by Shapiro-Wilk’s test. The statistical difference between groups (RD, SRD and SRD+Ca) was determined by one-way ANOVA followed by post-hoc Newman-Keuls' test. P values lower than 0.05 were considered to be statistically significant (SPSS 17.0 for Windows, SPSS INC. Chicago, Illinois).
Results
I Body Weight, Food Intake and Liver Weight
Figure 1A shows that throughout the experimental protocol (21 days) there was no significant difference in body weight in the experimental groups. Final food intake and liver weight did not differ between the groups (Figure 1B and 1C).
Figure 1: Body weight, food intake and liver weight of rats fed a control diet, sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca).
A) Body weight during the experimental period. Cannabis oil was administrated from the beginning of the experiment and during 3 weeks. Values are means for six animals per group, with standard errors represented by vertical bars. B) Food final intake and C) liver weight. Values are mean ± SEM (n= 6). Bars that do not share the same letter are significantly different, (P<0.05).
II Time Course of Systolic and Diastolic Blood Pressure
Table 2 shows the time course of systolic and diastolic blood pressure throughout the experimental period in the three dietary groups. In the first week of diet administration there were no significant differences in diastolic and systolic pressure between the experimental groups. From week 2 until the end, the animals SRD-fed showed a significant increase (P<0.05) in systolic and diastolic blood pressure compared with the RD-fed group. The administration of Cannabis oil (SRD+Ca) resulted in a significant reduction (P<0.05) in both parameters from the second week of administration, compared to the SRD group, reaching reference values.
Table
2:
Time course of systolic and diastolic blood pressure throughout the
experimental period in rats fed a reference diet (RD), sucrose-rich diet (SRD)
or SRD with cannabis oil (SRD+Ca).
|
|
RD |
SRD |
SRD+Ca |
|
Diastolic
Blood Pressure (mmHg) |
|
|
|
|
Week 1 (day 0 to 7) |
75.14 ± 1.51a |
76.572 ± 1,491a |
74.30 ± 1.07a |
|
Week
2 (day 8 to 15) |
74.27 ± 1.10b |
80.540 ± 1.550a |
76.05 ± 1.06b |
|
Week
3 (day 16 to 21) |
78.20 ± 1.34b |
87.909 ± 1.382a |
79.53 ± 1.05b |
|
Systolic
Blood Pressure (mmHg) |
|
|
|
|
Week 1 (day 0 to 7) |
111.17 ± 0.63a |
109.78 ± 1.36a |
108.52 ± 1.03a |
|
Week
2 (day 8 to 15) |
109.00 ± 0.75b |
120.28 ± 1.04a |
110.80 ± 1.72b |
|
Week
3 (day 16 to 21) |
119.58 ± 0.98b |
132.96 ± 0.19a |
117.67 ± 0.25b |
Values are expressed as mean ± SEM, n=6. Values in a
line that do not share the same superscript letter are significantly different
(P < 0.05) when one variable at a time was compared by one-way ANOVA
followed by Newman-Keuls´ test.
III Cannabis Effects in the Tetrad Assay
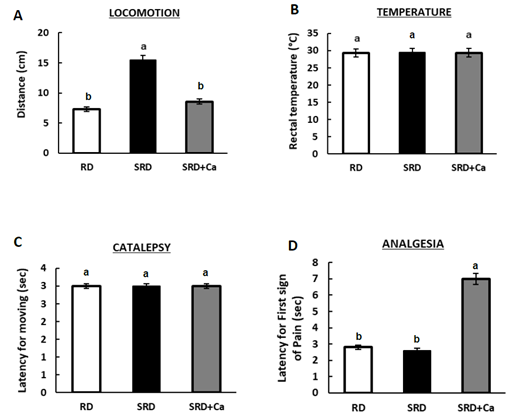
The cannabinoid activity of cannabis oil was evaluated by the tetrad of behavioural tests on rats. The tetrad includes the assessment of spontaneous activity (locomotion), immobility index (catalepsy), analgesia and changes in rectal temperature. Figure 2 shows that the incorporation of cannabis oil (SRD+Ca) significantly increased (P<0.05) analgesia. The locomotion, which was found to be increased in the SRD group, was significantly decreased (P<0.05) in the SRD+Ca group, reaching reference values. There were no significant differences in body temperature and catalepsy between the experimental groups.
Figure 2: Cannabinoid-Induced Tetrad test in rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis oil (SRD+Ca).
A) locomotion, B) temperature, C) catalepsy and D) analgesia.. Values are mean ± SEM (n= 6). Bars that do not share the same letter are significantly different, (P<0.05).
IV Serum Metabolites and Liver Damage Enzyme Activity
Table 3 shows that at the end of experimental protocol, both serum triglyceride, cholesterol and uric acid levels were significantly higher in SRD-fed rats compared with RD-fed rats. The administration of Cannabis oil (SRD+Ca) significantly decreased (P<0.05) serum Tg and cholesterol levels reaching reference values and uric acid levels was significantly reduced (P<0.05), although the values was still higher than RD group. No changes in serum glucose were recorded in the three dietary groups. Serum AST, ALT and AP levels were significantly increased (P<0.05) in SRD-fed rats compared to RD-fed rats. In the SRD+Ca group, AST and ALT enzymes significantly decreased (P<0.05) reached values similar to those observed in the RD group. AP enzyme was significantly reduced (P<0.05), although the values was still higher than RD group.
Table
3: Serum metabolites and liver damage enzyme activity in
rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis
oil (SRD+Ca).
|
|
RD |
SRD |
SRD+Ca |
|
Glucose (mM) |
7.71 ± 0.11a |
7.84 ± 0.07a |
7.62 ± 0.22a |
|
Triglyceride (mM) |
1.32 ± 0.09b |
3.08 ± 0.16a |
1.46 ± 0.11b |
|
Cholesterol
(mM) |
2.07 ± 0.07b |
2.63 ± 0.06a |
2.20 ± 0.11b |
|
Uric
acid (mM) |
49.20 ± 5.77c |
122.22 ± 8.83a |
72.49 ± 4.19b |
|
AST (U/L) |
19.56 ± 0.75b |
27.31 ± 0.82a |
21.54 ± 1.35b |
|
ALT (U/L) |
20.93 ± 1.39b |
29.92 ± 0.73a |
21.57 ± 1.97b |
|
AP (U/L) |
672.42 ± 54.61c |
1183.01 ± 25.07a |
1032.03 ± 11.95b |
Values are expressed as
mean ± SEM, n=6. Values in a line that do not share the same superscript letter
are significantly different (P < 0.05) when one variable at a time
was compared by one-way ANOVA followed by Newman-Keuls´ test.
V Metabolites and Damage Enzyme Activity in Liver
In (Figure 3) shows that in the liver, triglyceride and cholesterol content were significantly increased (P<0.05) in the SRD group compared to the RD group. In the SRD+Ca group, triglyceride content significantly decreased (P<0.05), although the values were still higher than RD group, while the cholesterol content returned to RD-group values. Furthermore, the SRD-fed rats had significantly higher (P<0.05) AST, ALT and AP hepatic levels, compared to RD-fed rats. In the SRD+Ca group, the values of AST and ALT hepatic enzymes returned to the values found in the RD-fed rats. AP hepatic enzyme was significantly reduced (P<0.05), although the values was still higher than RD group.
Figure 3: Metabolites hepatic and biomarkers of liver injure.
A) Triglyceride, B) cholesterol hepatic content, C) AST, D) ALT and E) AP liver enzymes. Values are mean ± SEM (n = 6). Bars that do not share the same letter are significantly different, (P<0.05).
Discussion
Cannabis preparations have been used for recreational and therapeutic purposes for thousands of years. These or some of their components activate an endogenous system of the body, the so-called endocannabinoid system, which is a complex physiological system that acts on metabolic pathways, which have not yet been explored. In this study, we present data showing that administration of a cannabis oil rich in CBD-THC (2:1) resulted of significant improvement in the several parameters present of MS. We observed that cannabis oil did not affect the body weight, food intake and liver weight. In this line, Assa-Glazer et al. observed that none of the plant extracts administrated in the study affected food consumption in mice a high-fat/cholesterol diet for 6 weeks [18]. During this period, cannabis extracts (CN1: rich in CBD, CN2: rich in THC, and CN6: similar concentrations of both phytocannabinoids) were administrated orally at a concentration of 5 mg/kg every 3 days. Wierucka-Rybak et al. revealed that CBD injections (3 mg/kg for 3 days) in rats fed high sucrose diet did not cause significant change in food intake and body weight [19].
However, other studies that investigated the impact of CBD or THC on food intake showed contradictory results. It has been reported that THC increases appetite and subsequent food intake [20]. Studies show that the hyperphagic effects of THC are mostly acute and are absent after a few days of administration. THC administered orally at a dose of 2 mg/kg, increased appetite 1 h after administration although the animals subsequently compensated for their hyperphagia, so that 24-h intakes were similar to controls [21]. On the other hand, Ignatowska-Jankowska et al. demonstrated that CBD (2.5 and 5 mg/kg) induced a decrease in body weight gain in rats, while other studies have shown no significant impact on food intake and body weight in mice and rats, respectively [22-24]. This contradiction may be explained by the concentrations of cannabinoids (i.e., THC and/or CBD) administered, time and route of administration used in the experiments.
Endocannabinoids are implicated in the pathogenesis of hypertension [25]. In our study, we show that the cannabis oil administration decreased systolic and diastolic blood pressure in dyslipidemic and hypertensive rats fed with a SRD. There are very few studies on the effects of cannabis or cannabinoids on hypertension. In this line, the majority of clinical evidence suggests that CBD dose not impact blood pressure in the populations assessed. In addition, there are very few clinical studies carried out in populations with high blood pressure, since CBD intervention studies have generally been carried out in healthy individuals or with non-cardiovascular conditions exposed to stress (cold, physical activity, public speaking), and more studies are required to determine if CBD supplementation can reduce blood pressure in patients with hypertension [26, 27]. On the other hand, in a study conducted with THC, they showed that inhalation of cannabis smoke from cigarettes (2.8% Δ9-THC) lowered blood pressure more durably in hypertensive subjects compared to normotensive subjects [28]. In animal studies, the effects of CBD on blood pressure were inconsistent and varied depending on the model of hypertension [29]. This is the first study evaluating the effect of cannabis oil (CBD-THC, 2:1) administration on systolic and diastolic blood pressure in SRD-fed hypertensive rats.
Cannabis sativa has been used for more than four centuries to treat a variety of medical conditions including pain. Cannabis plant as a whole has analgesic properties. In our study, we demonstrated that the administration of cannabis oil (CDC:THC 2:1) significantly increased analgesia in rats. In this line, Harris et al. demonstrated that THC administration produced robust analgesia equivalent to the full cannabis extract in male Sprague-Dawley rats [30]. Moore & Weerts showed a greater potency of oral THC to produce thermal antinociceptive effects in male rats, but oral CBD no antinociceptive effects were detected in the tail flick test, and in fact, increased pain sensitivity was observed after the highest dose of CBD (30 mg/kg) [31].
Cannabinoids have demonstrated beneficial effects on diabetes and lipid metabolism. In our study, we observed that cannabis oil administration to SRD-fed rats, significantly improved serum triglyceride, cholesterol and uric acid levels with no change in glucose levels. Enzymes AST and ALT are used as markers of liver injury. The cannabis oil administration presented reduction in these enzymes in serum and liver, together with an improvement in the hepatic content of triglyceride and cholesterol, suggesting a significant decrease in steatosis and liver damage, showing the hepatoprotective effect of cannabis oil used in present study. In this line, some studies suggest that the cannabinoids Δ9-THC and/or CBD increase HDL-C concentration, reduce total cholesterol and triglyceride in serum and decreases hepatic triglyceride accumulation and liver damage in other animal models [32-35]. Although none of them were performed in male Wistar rats fed with a SRD. In clinical studies, have shown that several cannabinoids (CBD, THC or THCV) has beneficial effects on biochemical parameters, such as blood glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides, and liver damage which are altered in diabetic patients [36, 37].
Cannabinoid-based therapies may also protect against diabetic complications. The results of this study provide new information on the beneficial effects of the administration of cannabis oil, with CBD and THC ratios of 2 to 1, useful in the treatment of several parameters included in the MS, among as hypertension, dyslipidemia and liver damage. However, to discover the complete mechanism behind these phenomena, further research is needed.
Conclusion
The present study demonstrates the beneficial effect of cannabis oil upon hypertension, dyslipidemia, mitigating steatosis and liver damage induced by a SRD. In addition, the analgesic effect of cannabis oil could be observed in SRD-fed rats. Furthermore, our results suggested that cannabis oil could serve as a new therapeutic agent to prevent or ameliorate metabolic disorders related included in MS.
Finally, although care must be taken when extrapolating these results from rats to humans, this animal model proves to be useful to study the mechanisms that determine the influence of nutrients/nutraceuticals in the development and management of metabolic diseases.
Acknowledgments
The authors would like to thank Silvia Rodríguez for their skillful technical assistance.
Author Agreement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Author Contributions
MEO: Funding acquisition, Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Supervision, Writing the manuscript. MVJ: Investigation, Methodology, Data curation, Formal analysis, Writing the manuscript. VD: Investigation, Data curation, Formal analysis, Writing the manuscript. CV: Investigation, Data curation, Formal analysis. DS: Investigation, Data curation, Formal analysis, Writing the manuscript. DA: Investigation, Data curation, Formal analysis, Writing the manuscript. MED: Conceptualization, Methodology, Data curation, Formal analysis, Writing the manuscript.
Conflicts of Interest
None.
Ethical Statement
This study protocol was reviewed and approved by the Institutional Ethics Committee of the Faculty of Biochemistry and Biological Sciences (Universidad Nacional del Litoral, Santa Fe, Argentina), approval number Acta 03/21.
Funding
This work was supported by Agencia Santafesina de Ciencia, Tecnología e Innovación from Argentina (PEICID-2021-003).
Abbreviations
ALT: Alanine Aminotransferase
AP: Alkaline Phosphatase
AST: Aspartate Aminotransferase
CBD: Cannabidiol
MS: Metabolic Syndrome
SRD: Sucrose-rich Diet
RD: Reference Diet
SRD+Ca: SRD+Cannabis Oil
THC: Tetrahydrocannabinol
Tg: Triglyceride
CBDVA: Cannabidivaric acid
CBDV: Cannabidivarin
CBDA: Cannabidiolic acid
CBGA: Cannabigerolic acid
CBG: Cannabigerol
CBD: Cannabidiol
THCV: Tetrahidrocannabivarin
THCVA: Tetrahidrocannabivarinic acid
CBN: Cannabinol
∆-9-THC: ∆-9-Tetrahidrocannabinol
∆-8-THC: ∆-8-Tetrahidrocannabinol
CBC: Cannabichromene
THCA: Tetrahydrocannabinolic acid
Article Info
Article Type
Research ArticlePublication history
Received: Thu 09, Mar 2023Accepted: Tue 04, Apr 2023
Published: Mon 17, Apr 2023
Copyright
© 2023 María Eugenia D’Alessandro. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2023.01.01
Author Info
Valentina Degrave Michelle Berenice Vega Joubert Cristian Vaccarini Daniela Sedan Darío Andrinolo María Eugenia D’Alessandro María Eugenia Oliva
Corresponding Author
María Eugenia D’AlessandroLaboratorio de Estudio de Enfermedades Metabólicas relacionadas con la Nutrición. Facultad de Bioquímica y Ciencias Biológicas. Universidad Nacional del Litoral. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Santa Fe, Argentina
Figures & Tables
Table 1: Composition of experimental diets.
|
SRDa |
|
|
Diet ingredients (g/kg diet) |
|
|
Sucrose |
580 |
|
Casein (vitamin free)b |
165 |
|
Corn oil |
105 |
|
Salt mix |
35 |
|
Vitamin mix |
10 |
|
Choline Chloride |
2 |
|
Methionine |
3 |
|
Fiber (cellulose) |
100 |
|
Energy (kJ/g) |
16.3 |
a The home-made SRD
experimental diet is based on the modified AIN-93M diet. b Casein was
provided by Saputo Molfino Hermanos S.A., Buenos Aires, Argentine.
Reference
diet (RD):
Rodent commercial diet (GEPSA FEED, Buenos Aires, Argentina) containing
(g/kg diet): carbohydrate (corn, sorghum, wheat, oats, barley) 427; protein
180; fat 39; fiber 55; minerals and vitamins 169; moisture 130; digestible
energy 12.5 kJ/g.
Table
2:
Time course of systolic and diastolic blood pressure throughout the
experimental period in rats fed a reference diet (RD), sucrose-rich diet (SRD)
or SRD with cannabis oil (SRD+Ca).
|
|
RD |
SRD |
SRD+Ca |
|
Diastolic
Blood Pressure (mmHg) |
|
|
|
|
Week 1 (day 0 to 7) |
75.14 ± 1.51a |
76.572 ± 1,491a |
74.30 ± 1.07a |
|
Week
2 (day 8 to 15) |
74.27 ± 1.10b |
80.540 ± 1.550a |
76.05 ± 1.06b |
|
Week
3 (day 16 to 21) |
78.20 ± 1.34b |
87.909 ± 1.382a |
79.53 ± 1.05b |
|
Systolic
Blood Pressure (mmHg) |
|
|
|
|
Week 1 (day 0 to 7) |
111.17 ± 0.63a |
109.78 ± 1.36a |
108.52 ± 1.03a |
|
Week
2 (day 8 to 15) |
109.00 ± 0.75b |
120.28 ± 1.04a |
110.80 ± 1.72b |
|
Week
3 (day 16 to 21) |
119.58 ± 0.98b |
132.96 ± 0.19a |
117.67 ± 0.25b |
Values are expressed as mean ± SEM, n=6. Values in a
line that do not share the same superscript letter are significantly different
(P < 0.05) when one variable at a time was compared by one-way ANOVA
followed by Newman-Keuls´ test.
Table
3: Serum metabolites and liver damage enzyme activity in
rats fed a reference diet (RD), sucrose-rich diet (SRD) or SRD with cannabis
oil (SRD+Ca).
|
|
RD |
SRD |
SRD+Ca |
|
Glucose (mM) |
7.71 ± 0.11a |
7.84 ± 0.07a |
7.62 ± 0.22a |
|
Triglyceride (mM) |
1.32 ± 0.09b |
3.08 ± 0.16a |
1.46 ± 0.11b |
|
Cholesterol
(mM) |
2.07 ± 0.07b |
2.63 ± 0.06a |
2.20 ± 0.11b |
|
Uric
acid (mM) |
49.20 ± 5.77c |
122.22 ± 8.83a |
72.49 ± 4.19b |
|
AST (U/L) |
19.56 ± 0.75b |
27.31 ± 0.82a |
21.54 ± 1.35b |
|
ALT (U/L) |
20.93 ± 1.39b |
29.92 ± 0.73a |
21.57 ± 1.97b |
|
AP (U/L) |
672.42 ± 54.61c |
1183.01 ± 25.07a |
1032.03 ± 11.95b |
Values are expressed as
mean ± SEM, n=6. Values in a line that do not share the same superscript letter
are significantly different (P < 0.05) when one variable at a time
was compared by one-way ANOVA followed by Newman-Keuls´ test.

A) Body weight during the experimental period. Cannabis oil was administrated from the beginning of the experiment and during 3 weeks. Values are means for six animals per group, with standard errors represented by vertical bars. B) Food final intake and C) liver weight. Values are mean ± SEM (n= 6). Bars that do not share the same letter are significantly different, (P<0.05).
A) locomotion, B) temperature, C) catalepsy and D) analgesia.. Values are mean ± SEM (n= 6). Bars that do not share the same letter are significantly different, (P<0.05).

A) Triglyceride, B) cholesterol hepatic content, C) AST, D) ALT and E) AP liver enzymes. Values are mean ± SEM (n = 6). Bars that do not share the same letter are significantly different, (P<0.05).
References
1.
Lemieux
I, Després JP (2020) Metabolic Syndrome: Past, Present and Future. Nutrients
12: 3501. [Crossref]
2.
Sherling
DH, Perumareddi P, Hennekens CH (2017) Metabolic Syndrome. J Cardiovasc.
Pharmacol Ther 22: 365-367. [Crossref]
3.
Lombardo
YB, Drago S, Chicco A, Fainstein Day P, Gutman R et al. (1996) Long-term
administration of a sucrose-rich diet to normal rats: relationship between
metabolic and hormonal profiles and morphological changes in the endocrine
pancreas. Metabolism 45: 1527-1532. [Crossref]
4.
Toop
CR, Gentili S (2016) Fructose Beverage Consumption Induces a Metabolic Syndrome
Phenotype in the Rat: A Systematic Review and Meta-Analysis. Nutrients.
8: 577. [Crossref]
5.
Wei
Y, Wang D, Topczewski F, Pagliassotti MJ (2007) Fructose-mediated stress
signaling in the liver: implications for hepatic insulin resistance. J Nutr
Biochem 18: 1-9. [Crossref]
6.
Chicco
AG, D’Alessandro ME, Hein GJ, Oliva ME, Lombardo YB (2009) Dietary chia seed
(Salvia hispanica L.) rich in a-linolenic acid improves adiposity and
normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic
rats. Br J Nutr 101: 41-50. [Crossref]
7.
Rossi
AS, Oliva ME, Ferreira MR, Chicco A, Lombardo YB (2013) Dietary chia seed
induced changes in hepatic transcription factors and their target lipogenic and
oxidative enzyme activities in dyslipidaemic insulin-resistant rats. Br J
Nutr 109: 1617-1627. [Crossref]
8.
Bonini
SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G et al. (2018) Cannabis
sativa: A comprehensive ethnopharmacological review of a medicinal plant with a
long history. J Ethnopharmacol 227: 300-315. [Crossref]
9.
Bridgeman
MB, Abazia DT (2017) Medicinal Cannabis: History, Pharmacology, and
Implications for the Acute Care Setting. P T 42: 181-188. [Crossref]
10. Hussain T, Jeena G,
Pitakbut T, Vasilev N, Kayser O (2021) Cannabis sativa research trends,
challenges, and new-age perspectives. iScience 24: 103391. [Crossref]
11. Radwan MM, Chandra
S, Gul S, ElSohly MA (2021) Cannabinoids, Phenolics, Terpenes and Alkaloids of
Cannabis. Molecules 26: 2774 [Crossref]
12. Bielawiec P,
Harasim Symbor E, Chabowski A (2020) Phytocannabinoids: Useful Drugs for the
Treatment of Obesity? Special Focus on Cannabidiol. Front Endocrinol
(Lausanne) 11: 114-125. [Crossref]
13. Gruden G,
Barutta F, Kunos G, Pacher P (2016) Role of the endocannabinoid system in
diabetes and diabetic complications. Br. J. Pharmacol. 173: 1116-1127. [Crossref]
14. Horváth B,
Mukhopadhyay P, Haskó G, Pacher P (2012) The Endocannabinoid System and
Plant-Derived Cannabinoids in Diabetes and Diabetic Complications. Am. J.
Pathol 180: 432-442. [Crossref]
15. Sedan D, Vaccarini
C, Demetrio P, Morante M, Montiel R et al. (2023) Cannabinoid Content in
Cannabis Flowers and Homemade Cannabis-Based Products Used for Therapeutic
Purposes in Argentina. Cannabis Cannabinoid Res 8: 197-206. [Crossref]
16. Creus A, Benmelej
A, Villafañe N, Lombardo YB (2017) Dietary Salba (Salvia hispanica L) improves
the altered metabolic fate of glucose and reduces increased collagen deposition
in the heart of insulin-resistant rats. Prostaglandins Leukot Essent Fatty
Acids 121: 30-39. [Crossref]
17. Metna Laurent M,
Mondesir M, Grel A, Vallee M, Piazza PV (2017) Cannabinoid-Induced Tetrad in
Mice. Curr. Protoc Neurosci. 80: 9.59.1-9.59.10. [Crossref]
18. Assa Glazer T,
Gorelick J, Sela N, Nyska A, Bernstein N et al. (2020) Cannabis Extracts
Affected Metabolic Syndrome Parameters in Mice Fed High-Fat/Cholesterol Diet. Cannabis
Cannabinoid Res. 5: 202-214. [Crossref]
19. Wierucka Rybak M,
Wolak M, Bojanowska E (2014) The effects of letin in combination with a
cannabinoid receptor 1 antagonist, AM 251, or cannabidiol on food intake and
body weight in rats fed a high-fat or free-choice high sugar diet. J Physiol
Pharmacol 65: 487-496. [Crossref]
20. Fearby N, Penman S,
Thanos P (2022) Effects of ∆9-Tetrahydrocannibinol (THC) on Obesity at
Different Stages of Life: A Literature Review. Int J Environ Res Public
Health 19: 3174-3202. [Crossref]
21. Williams CM, Rogers
PJ, Kirkham TC (1998) Hyperphagia in Pre-Fed Rats Following Oral Δ9 -THC. Physiol
Behav 65: 343-346. [Crossref]
22. Ignatowska
Jankowska B, Jankowski MM, Swiergiel AH (2011) Cannabidiol decreases body
weight gain in rats: Involvement of CB2 receptors. Neurosci Lett 490:
82-84 [Crossref]
23. Riedel G, Fadda P,
McKillop Smith S, Pertwee RG, Platt B et al. (2009) Synthetic and plant-derived
cannabinoid receptor antagonists show hypophagic properties in fasted and
non-fasted mice. Br J Pharmacol 156: 1154-1166. [Crossref]
24. Wiley JL, Burston
JJ, Leggett DC, Alekseeva OO, Razdan RK et al. (2005) CB1 cannabinoid
receptor-mediated modulation of food intake in mice. Br J Pharmacol 145:
293-300. [Crossref]
25. Malinowska B,
Baranowska Kuczko M, Schlicker E (2012) Triphasic blood pressure responses to
cannabinoids: do we understand the mechanism? Br J Pharmacol 165:
2073-2088. [Crossref]
26. Azari EK, Kerrigan
A, O’Connor A (2020) Naturally Occurring Cannabinoids and their Role in
Modulation of Cardiovascular Health. J Diet Suppl 17: 625-650. [Crossref]
27. Jadoon KA, Tan GD,
O’Sullivan SE (2017) A single dose of cannabidiol reduces blood pressure in healthy
volunteers in a randomized crossover study. JCI Insight 2: e93760. [Crossref]
28. Baranowska Kuczko
M, Kozłowska H, Kloza M, Sadowska O, Kozłowski M et al. (2020) Vasodilatory
effects of cannabidiol in human pulmonary and rat small mesenteric arteries:
modification by hypertension and the potential pharmacological opportunities. J
Hypertens 38: 896-911. [Crossref]
29. Remiszewski P,
Jarocka Karpowicz I, Biernacki M, Jastrząb A, Schlicker E et al. (2020) Chronic
Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with
Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma
Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int J Mol Sci
21: 1295. [Crossref]
30. Harris HM, Rousseau
MA, Wanas AS, Radwan MM, Caldwell S et al. (2019) Role of Cannabinoids and
Terpenes in Cannabis-Mediated Analgesia in Rats. Cannabis Cannabinoid Res
4: 177-182. [Crossref]
31. Moore CF, Weerts EM
(2022) Cannabinoid tetrad effects of oral Δ9-tetrahydrocannabinol (THC) and
cannabidiol (CBD) in male and female rats: sex, dose-effects and time course
evaluations. Psychopharmacology (Berl) 239: 1397-1408. [Crossref]
32. Comelli F, Bettoni
I, Colleoni M, Giagnoni G, Costa B (2009) Beneficial effects of a Cannabis
sativa extract treatment on diabetes-induced neuropathy and oxidative stress. Phytother
Res 23: 1678-1684. [Crossref]
33. Silvestri C, Di
Marzo V (2013) The Endocannabinoid System in Energy Homeostasis and the
Etiopathology of Metabolic Disorders. Cell Metab 17: 475-490. [Crossref]
34. Wang Y,
Mukhopadhyay P, Cao Z, Wang H, Feng D et al. (2017) Cannabidiol attenuates
alcohol-induced liver steatosis, metabolic dysregulation, infammation and
neutrophil-mediated injury. Sci. Rep 21: 12064. [Crossref]
35. Wargent ET, Zaibi
MS, Silvestri C, Hislop DC, Stocker CJ et al. (2013) The cannabinoid D9
-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse
models of obesity. Nutr Diabetes 3: e68. [Crossref]
36. Adejumo AC, Alliu S, Ajayi TO, Adejumo KL, Adegbala OM et al. (2017) Cannabis use is associated with reduced prevalence of non-alcoholic fatty liver disease: A cross-sectional study. PLoS One 12: e0176416. [Crossref]
37. Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C et al. (2016) Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 39: 1777-1786. [Crossref]