Differential Expression of Blood Group Precursor Antigen in Human Breast Cancer Tissue
A B S T R A C T
There is a pressing need for biomarkers for targeted immunotherapy against breast cancer (BCA), the leading cause of cancer death in women. Previously, a blood group precursor O-core epitope gpC1 was found to be highly expressed in breast circulating tumor cells (BCTCs) and BCA cell lines with cancer stem cell (BCSC) features. In this pilot study, the breast tissue distribution of gpC1 was examined using tissue microarrays (TMAs). Notably, gpC1 positive cells were detected in the major histological types of neoplastic breast tissues. Conversely, none of the breast tissues derived from subjects without BCA were gpC1 positive. Thus, gpC1 expression seems to be tumor-specific but not histological type-dependent, reflecting perhaps its characteristics as a conserved epitope of oncofetal blood group precursor antigens.
Keywords
Blood group precursors, breast cancer, circulating tumor cells, cancer stem cells, glycan marker, tissue microarray
Introduction
Worldwide, breast cancer (BCA) is the most frequent malignancy and the leading cause of cancer death in women [1, 2]. It is a heterogeneous disease at the molecular level, and the treatment concepts have evolved to include biologically directed therapies [1, 3]. There is an urgent need for biomarkers that can provide information about clinical and pathological features and prognosis to guide treatment stratification [4]. It is well known that abnormal glycosylation is involved in virtually every cancer type [5, 6]. Glycan markers take advantage of surface-exposed and easily accessible cellular features and may be important potential BCA biomarkers in the era of precision therapy. Previously, we investigated the expression of a blood group precursor O-core glyco-epitope gpC1 in BCA at the cellular level and demonstrated that gpC1 is frequently expressed by a number of human BCA cell lines, as well as by breast circulating tumor cells (BCTCs) in stage IV metastatic BCA patients [5-7]. Interestingly, in a patient with advanced triple-negative BCA, 92.5% of CTCs (37/40 CTCs) were found to be gpC1 positive [5].
The high expression rate of gpC1 in BCA suggests this blood group precursor antigen may be a BCA biomarker, and the strikingly high percentage of gpC1 positive CTCs in the advanced stage of triple-negative BCA indicate gpC1 is potentially associated with aggressive tumor behaviour. Hence, in the present study, we further investigated the expression of glyco-epitope gpC1 in tissue microarrays from BCA patients who had different histological tumor types classified at various pathological stages.
Methods and Materials
A key immunological probe of this investigation was the anti-tumor glycan monoclonal antibody (mAb), G1, which opposes epiglycanin, the major sialomucin glycoprotein (~500 kDa) of murine mammary adenocarcinoma TA3 cells and has been shown to specifically bind human carcinoma-associated antigen in vitro [8, 9]. As has been seen with mAb AE-3, G1 is highly specific for glyco-epitope gpC1 [6]. De-identified human BCA tissue microarrays were obtained from the Cooperative Human Tissue Network (CHTN), mid-Atlantic division (Charlottesville, VA, USA). The immunohistochemistry (IHC) study was performed on formalin-fixed, paraffin-embedded tissue microarray sections using mAb G1. An independent, blinded evaluation was performed by qualified pathologists using three parallel staining results. Statistical analyses were performed using SAS Survey Procedures (SAS 9.4, SAS Institute Inc, Cary, NC, USA). Fisher's exact test was used for two-group comparisons.
Results and Discussion
The IHC study was performed on 64 specimens of the tissue microarray; four samples were excluded because there was not enough tissue for interpretation, and eight samples served as controls. A total of 52 breast tissue specimens were included in the analysis (Table 1). As shown in (Table 1), there were 13 non-neoplastic specimens: six were from patients without breast carcinoma (NB-NC), and seven were from patients diagnosed with breast carcinoma (NB C). No gpC1 expression was observed in the NB-NC specimens (as indicated by uniformly negative G1 staining). The tissues from the NB-NC and NB-C group showed a gpC1 expression frequency of 2/13 (15.4%), and only one specimen demonstrated focal strong positive staining. The 39 remaining neoplastic specimens exhibited a 26/39 (66.7%) gpC1 positive rate, of which 12/39 (30.8%) showed diffuse positive or focal strong positive patterns. Compared to the non-neoplastic specimens, BCA neoplastic specimens had a higher rate of gpC1 expression (P = 0.0028).
Table 1: Results of breast tissue microarray immunohistochemical analysis of gpC1 expression.
|
|
|
gpC1 Positive (%) |
gpC1 Negative (%) |
gpC1 Diffuse or focal strong positive (%) |
|
Non-neoplastic |
NB-NC (n = 6) |
0(0) |
6(100%) |
0(0%) |
|
breast tissue |
NB-C (n = 7) |
2(28.6%) |
5(71.4%) |
1(14.3%) |
|
|
Non-neoplastic total |
2(15.4%) |
11(84.6%) |
1(7.7%) |
|
Neoplastic |
DCIS-L (n = 7) |
5(71.4%) |
2(28.6%) |
2(28.6%) |
|
breast tissue |
DCIS-H (n = 7) |
4(57.1%) |
3(42.9%) |
2(28.6%) |
|
|
DCIS total |
9(64.3%) |
5(35.7%) |
4(28.6%) |
|
|
IDC-L (n = 6) |
5(83.3%) |
1(16.7%) |
3(50%) |
|
|
IDC-H (n = 7) |
6(85.7%) |
1(14.3%) |
2(28.6%) |
|
|
IDC total |
11(84.6%) |
2(15.4%) |
5(38.5%) |
|
|
ILC (n = 5) |
3(60%) |
2(40%) |
2(40%) |
|
|
LNM (n = 7) |
3(42.9%) |
4(57.1%) |
1(14.3%) |
|
|
Neoplastic total |
26(66.7%) |
13(33.3%) |
12(30.8%) |
NB-NC: Non-neoplastic breast tissue from patients without breast carcinoma; NB-C: Non-neoplastic breast tissue from breast carcinoma patients; DCIS-L: Ductal carcinoma in situ, low grade; DCIS-H: Ductal carcinoma in situ, high grade; IDC-L: Invasive ductal carcinoma, grade 1 or 2; IDC-H: Invasive ductal carcinoma, grade 3; ILC: Invasive lobular carcinoma; LNM: Breast carcinoma metastatic to regional lymph nodes.
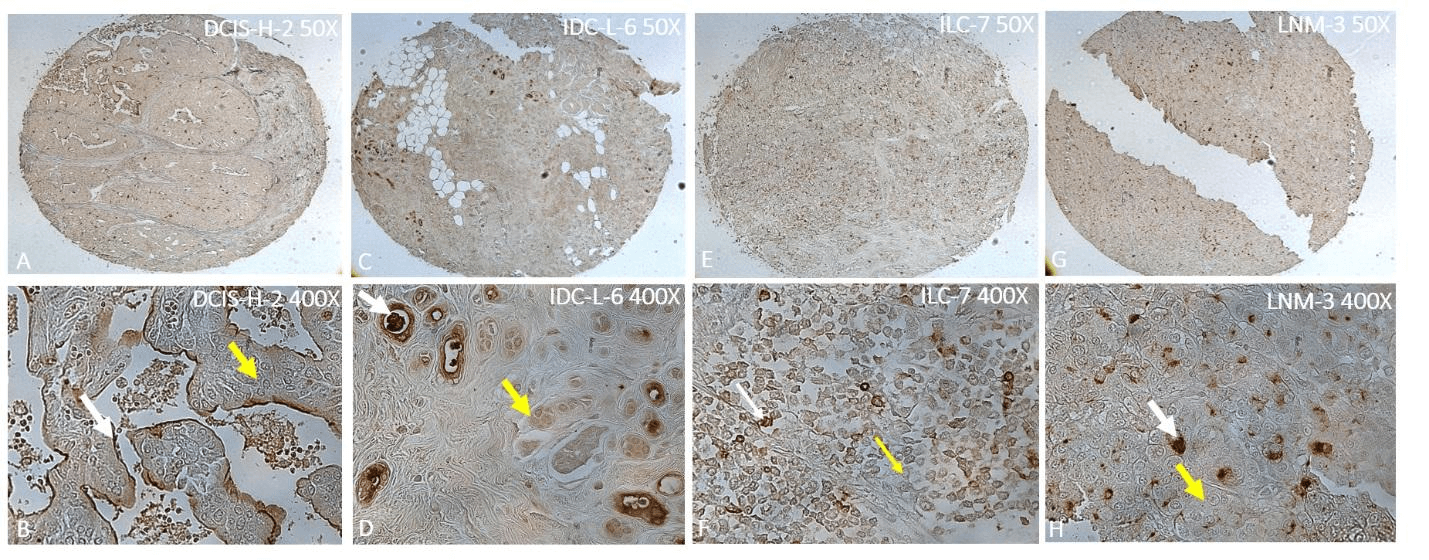
Figure 1: Immunohistochemical study of gpC1 expression in primary and metastatic BCA tissue with different pathological grades and histological types by antibody G1. White arrows show positive staining, and yellow arrows show negative staining. A) Diffuse positive staining in high-grade DCIS (DCIS-H). B) Positive vs. negative staining in an area of DCIS-H. C) Diffuse positive staining in low-grade IDC (IDC-L) (grade 1 and 2). D) Local strong positive vs. negative staining in an area of IDC-L. E) Diffuse positive staining in ILC. F) Positive vs. negative staining in an area of ILC. G) Diffuse positive staining in LNM. H) Positive vs. negative staining in an area of LNM.
We examined the pattern of gpC1 expression in neoplastic specimens with different pathological grades and histological types. As shown in (Figure 1), gpC1 was overexpressed in a subset of BCA cells within each type of positive specimens, while other BCA cells did not stain. Most gpC1 positive cells were cytoplasmic or membranous stained. Notably, gpC1-positive cancer cells and negative cancer cells co-existed in most of the BCA tissue specimens with the latter as the predominant cell populations while the former appeared like “seeds” in the “lawn” of negatively stained cancer tissues. Clarke et al. have demonstrated a cancer stem cell (CSC) model in solid tumors [10]. BCSCs are a small subset of BCA cells and thought to have a central role in the initiation and progression of BCA and in the clinical response to therapy. The BCSC related mutation pathways have been reported to correlate with metastases and the markers CD44/CD24 and ALDH1 have been widely used; however, their expression is not always consistent [11, 12]. The gpC1, which is a blood group precursor-based oncofetal antigen, may be explored as a BCSC biomarker candidate, and it showed no cross-reactivity with normal breast tissue in our study [5-7]. In fact, the selective gpC1 expression in NB-C specimens may indicate gpC1 could serve as a cancer-specific target for use in early detection of BCA even before neoplastic changes.
In conclusion, these findings suggest the gpC1 blood group precursor-based oncofetal antigen may be useful in identifying a subset of BCA cells. Further investigation of gpC1 expression level, its relationship with BCA progression, and comparisons with traditional BCSC markers are needed. Further exploration of glycan markers for BCTCs/BCSCs is likely to provide new insight into precision medicine and targeted immunotherapy of BCA.
Acknowledgements
The authors acknowledge the Cooperative Human Tissue Network (CHTN), mid-Atlantic division (Charlottesville, VA, USA) for breast cancer tissue microarrays. This work was supported in part by US Government grants U01CA128416 (NIH/NCI), 1R21AI124068 (NIH), 1R21DA046144 (NIH), and PR170128 (CDMRP) to DW. The content is solely the responsibility of the author and does not necessarily represent the official views of the funding agents.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 22, Jul 2020Accepted: Wed 05, Aug 2020
Published: Fri 14, Aug 2020
Copyright
© 2023 Denong Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.IJCST.2020.01.04
Author Info
Corresponding Author
Denong WangTumor Glycomics Laboratory, SRI International Biosciences Division, Menlo Park, California, USA
Figures & Tables
Table 1: Results of breast tissue microarray immunohistochemical analysis of gpC1 expression.
|
|
|
gpC1 Positive (%) |
gpC1 Negative (%) |
gpC1 Diffuse or focal strong positive (%) |
|
Non-neoplastic |
NB-NC (n = 6) |
0(0) |
6(100%) |
0(0%) |
|
breast tissue |
NB-C (n = 7) |
2(28.6%) |
5(71.4%) |
1(14.3%) |
|
|
Non-neoplastic total |
2(15.4%) |
11(84.6%) |
1(7.7%) |
|
Neoplastic |
DCIS-L (n = 7) |
5(71.4%) |
2(28.6%) |
2(28.6%) |
|
breast tissue |
DCIS-H (n = 7) |
4(57.1%) |
3(42.9%) |
2(28.6%) |
|
|
DCIS total |
9(64.3%) |
5(35.7%) |
4(28.6%) |
|
|
IDC-L (n = 6) |
5(83.3%) |
1(16.7%) |
3(50%) |
|
|
IDC-H (n = 7) |
6(85.7%) |
1(14.3%) |
2(28.6%) |
|
|
IDC total |
11(84.6%) |
2(15.4%) |
5(38.5%) |
|
|
ILC (n = 5) |
3(60%) |
2(40%) |
2(40%) |
|
|
LNM (n = 7) |
3(42.9%) |
4(57.1%) |
1(14.3%) |
|
|
Neoplastic total |
26(66.7%) |
13(33.3%) |
12(30.8%) |
NB-NC: Non-neoplastic breast tissue from patients without breast carcinoma; NB-C: Non-neoplastic breast tissue from breast carcinoma patients; DCIS-L: Ductal carcinoma in situ, low grade; DCIS-H: Ductal carcinoma in situ, high grade; IDC-L: Invasive ductal carcinoma, grade 1 or 2; IDC-H: Invasive ductal carcinoma, grade 3; ILC: Invasive lobular carcinoma; LNM: Breast carcinoma metastatic to regional lymph nodes.
References
- Nadia Harbeck, Frédérique Penault Llorca, Javier Cortes, Michael Gnant, Nehmat Houssami et al. (2019) Breast cancer. Nat Rev Dis Primers 5: 66. [Crossref]
- Lindsey A Torre, Freddie Bray, Rebecca L Siegel, Jacques Ferlay, Joannie Lortet Tieulent et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108. [Crossref]
- Nora Pashayan, Antonis C Antoniou, Urska Ivanus, Laura J Esserman, Douglas F Easton et al. (2020) Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. [Crossref]
- Anna Kazarian, Oleg Blyuss, Gergana Metodieva, Aleksandra Gentry Maharaj, Andy Ryan et al. (2017) Testing breast cancer serum biomarkers for early detection and prognosis in pre-diagnosis samples. Br J Cancer 116: 501-508. [Crossref]
- Denong Wang, Xiaohe Liu, Ben Hsieh, Richard Bruce, George Somlo et al. (2015) Exploring Glycan Markers for Immunotyping and Precision-targeting of Breast Circulating Tumor Cells. Arch Med Res 46: 642-650. [Crossref]
- Denong Wang, Jin Tang, Shaoyi Liu, Jiaoti Huang (2015) Carbohydrate Microarrays Identify Blood Group Precursor Cryptic Epitopes as Potential Immunological Targets of Breast Cancer. J Immunol Res 2015: 510810. [Crossref]
- Denong Wang (2017) Unraveling Sugar Chain Signatures of the "Seeds" of Tumor Metastasis. J Proteomics Bioinform 10: e31. [Crossref]
- J F Codington, B H Sanford, R W Jeanloz (1972) Glycoprotein coat of the TA3 cell. Isolation and partial characterization of a sialic acid containing glycoprotein fraction. Biochemistry 11: 2559-2564. [Crossref]
- T Thingstad, S Haavik, K Hansen, K Sletten, J F Codington et al. (1998) Human carcinoma-associated antigen (HCA), isolated from the endometrial carcinoma cell line KLE-1 and ascitic fluid of a patient with ovarian carcinoma; comparison with epiglycanin. Eur J Pharm Sci 6: 121-129. [Crossref]
- Michael F Clarke, John E Dick, Peter B Dirks, Connie J Eaves, Catriona H M Jamieson et al. (2006) Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 66: 9339-9344. [Crossref]
- Vanessa Marchesi (2013) Breast cancer: Mutations in breast cancer stem cells correlate with metastases. Nat Rev Clin Oncol 10: 546. [Crossref]
- Wenzhe Li, Huailei Ma, Jin Zhang, Ling Zhu, Chen Wang et al. (2017) Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci Rep 7: 13856. [Crossref]
