Low Level of Knowledge of Atrial Fibrillation and Anticoagulant Treatment among Patients with Atrial Fibrillation Scheduled for Cardiac Surgery
A B S T R A C T
Objective: We investigated the knowledge about atrial fibrillation (AF) and oral anticoagulants (OACs) in AF patients scheduled for cardiac surgery compared with nonsurgical AF patients.
Methods: We recruited 144 consecutive patients with documented AF scheduled for cardiac surgery on admission (aged 68.9±8.4, male 60.4 %). The control group represented 200 age- and sex-matched AF patients without indications for surgery. Using the validated Jessa AF Knowledge Questionnaire (JAKQ), we tested their knowledge of AF and the use of OAC.
Results: The mean score on the JAKQ was 47±20 % in the surgery group and 59±18 % in the control group (p<0.001), without any questions in which the former group scored better. A higher level of knowledge was observed in patients taking vitamin K antagonists (VKA) in the past, and individuals free of heart failure, previous stroke, or peripheral artery disease. Patients had poor knowledge of the safety issues, including 27.5% of surgical patients who knew about possible painkillers use during anticoagulation compared with 43.8% in the control group (p=0.002). Patients scheduled for valvular surgery (n=88, 61.5%) scored better compared with those (n=26, 18.2%) for coronary artery bypass graft (CABG) surgery (49±19% vs. 35±18 %, p=0.002 respectively).
Conclusion: The level of knowledge about AF and its treatment, including the safety issues, is poor among AF patients admitted for cardiac surgery. More educational efforts should be taken in this vulnerable patient subset.
Keywords
Atrial fibrillation, questionnaire, cardiac surgery, knowledge, oral anticoagulation
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia, is associated with high mortality and morbidity, in particular the risk of ischemic stroke and systemic thromboembolism [1-5]. AF remains the most frequent arrhythmia encountered in 10-15% of patients undergoing cardiac surgery. Advanced age is a risk factor of AF, which is in line with an increasing number of elderly patients referred for surgical procedures [6]. Attaran et al. showed that among patients undergoing any cardiac operation, 1925 (13.5%) had chronic AF preoperatively [7]. Preoperative AF increases the risk of developing postoperative complications leading to death and prolonged hospital stay [7, 8]. It has been demonstrated that preoperative AF increased the risk of stroke by twofold (9%) [9]. Ngaage et al. reported that the late cardiac death rate in the preoperative AF group undergoing coronary artery bypass graft (CABG) surgery was 2.8 times higher than in the sinus rhythm group [10].
Most of AF patients require life-long anticoagulation with vitamin K antagonists (VKA) or preferably, non-vitamin K antagonist oral anticoagulants (NOACs) [11]. The current European Society of Cardiology (ESC) guidelines regarding the management of AF together with the 2018 European Heart Rhythm Association (EHRA) practical guide highlight that patients’ education is important for effectiveness and safety of anticoagulation therapy (class I recommendation), especially among patients undergoing valve replacement surgery [11, 12]. The annual rate of prosthetic valve thrombosis ranges from 0.1% to 5.7%, with higher rates observed with older valve types and in the early perioperative period, especially at the time of subtherapeutic anticoagulation [13].
Evaluation of the patients’ knowledge is of key importance in improving the quality of anticoagulation therapy and patient care. A few questionnaires have been published as useful tools for assessing AF patients’ knowledge, including the Jessa AF Knowledge Questionnaire (JAKQ) [14-20]. To our knowledge, there have been no such reports in AF patients undergoing cardiac surgery. We hypothesized that AF patients who awaited cardiac surgery, in particular those prior to cardiac valve replacement, supported by healthcare providers, are more willing to increase their overall knowledge and to be aware of elevated risk of adverse events, both thromboembolic and bleeding, following a major cardiac surgical procedure. We sought to assess the knowledge using the JAKQ among patients with AF admitted to hospital for scheduled heart surgery.
Materials and Methods
I Patients
In this case-control study, we recruited 156 patients with a documented AF admitted to a specialist cardiology center, the John Paul II Hospital in Kraków (Poland) for scheduled heart surgery from March 2017 to April 2018. Patients were admitted to the hospital for elective CABG surgery, heart valve surgery, or other cardiac surgery. All included patients were above 18 years of age and provided oral consent to participate in this study. Patients were individually asked to complete the questionnaire on admission to the hospital, and subsequently any concerns were discussed with a treating physician. The control group comprised of 200 patients with AF matched for age and gender by frequency, who were recruited at the same time in the same centre. The main causes of hospitalizations among eligible patients were exacerbation of heart failure or diagnostic workup of vascular disease. The study was approved by the local Ethical Committee.
II Questionnaire
We used the JAKQ after having obtained formal consent and making its translation into Polish. JAKQ was developed by Desteghe et al. to test the patients’ knowledge about arrhythmia, treatment, and their capability for self-management [18]. The questionnaire contained 16 multiple-choice questions with only one correct answer, as described previously [18-20]. Sections regarding particular medications, which were never used by the patients recruited (e.g., questions regarding NOACs in patients on VKA), were excluded from the final analysis. The patients who used both VKA and NOAC completed both sections. The time needed to complete the questionnaire was also recorded. A total score was calculated from all completed questions and displayed as the percentage.
III Clinical Data
Demographic and clinical data were obtained from medical history. From the medical records, we retrieved information about co-morbidities. Valve replacement denoted the implantation of a cardiac mechanical valve prosthesis. Mitral stenosis was defined according to the ESC guidelines and classified from mild to severe [21]. We also documented the type of OACs administered in the preceding period, the dosing regimen, and the history of VKA treatment in the past. We collected data about bleeding episodes categorized as severe bleeding episodes, gingival bleeding, nose bleeding, and easy bruising. Major bleeding events were defined according to Schulman et al. [22]. Patients in the surgery group were also asked about the level of education.
IV Statistics
Continuous variables were presented as means (standard deviation) or median (interquartile range) as appropriate. The Kolmogorov-Smironov test was used to determine the normal distribution of variables. Categorical variables were reported as numbers and percentages. The chi-square test was used to compare categorical variables. The ANOVA or Kruskal-Wallis tests for continuous variables were used to assess differences between the groups. Multivariate logistic regression analysis was conducted to determine which variables were significant predictors of correct responses to selected questions. Backward logistic regression was applied. Statistical analyses were performed using JMP 14.0 (SAS Institute Inc. Cary, NC, USA, 2018). P-values <0.05 were accepted as statistically significant.
Results
I Patient Characteristics
Due to errors in questionnaires, mainly providing 2 or 3 responses to one question, and incomplete clinical data, 12 patients (7.7%) were excluded from the final analysis, which involved 144 patients from the surgery group, including 105 (72.9%) scheduled for valvular surgery alone or in combination with CABG and 200 matched control patients (Table 1 and Supplementary Table 1). Men were overrepresented in the CABG subgroup. In the control group, initially, 4.9% of questionnaires were excluded due to the same reasons as in the surgery group. Almost all patients had stopped their regular anticoagulant treatment before admission, and bridging anticoagulation with low-molecular-weight-heparins (LMWH) was introduced.
In the surgery group, there were 48 patients (33.3%) treated with VKA, 26 patients (18.1%) on warfarin, and 22 patients (15.3%) on acenocoumarol. 78 patients (54.1%) were treated with NOACs. As few as 12 patients (8.3%) received low-molecular-weight-heparin at therapeutic or intermediate doses. Four patients (4.1%) did not receive any antithrombotic treatment at enrollment. Two patients did not remember the name of the anticoagulant. In the control group there were 80 AF patients (40%) treated with VKA, including 39 (19.5%) on warfarin and 41 (20.5%) on acenocoumarol, while 103 patients (51.5%) received NOACs, 5 (2.5%) received a LMWH, and 10 (5%) had no antithrombotic treatment. None in the surgery group patients took acetylsalicylic acid in monotherapy as stroke prevention in contrast to two subjects in the control group (1%).
The most common NOAC in the surgery and in the control, group was rivaroxaban (28.5% vs 27%, p=0.9, respectively), with subsequent dabigatran (22.2% vs and 23.5%, p=0.8) and apixaban (3.5% vs 0.5%, p=0.08). Both groups had a similar prevalence of most comorbidities and differed significantly only in the proportion of patients with PAD and diabetes (Table 1). Among patients in the surgery group who were asked about the level of education 68 patients (47%) achieved secondary education, 59 patients had primary education (41%), and 17 patients had university education (11.8%). Patients in the surgery group needed more time to complete the JAKQ questionnaire (13 vs. 10 minutes, p=0.03).
Table 1: Characteristics of the study population.
|
|
Surgery group (n=144) |
Control group (n=200) |
p-value |
Valve surgery (n=88) |
Other surgery (n=55) |
p-value |
|
Age, year |
68.9±8.4 |
68.4±11.7 |
0.6 |
68.8±9.0 |
68.9±7.3 |
0.9 |
|
Male gender, n (%) |
87 (60.4) |
118 (59.0) |
0.8 |
45 (51.1) |
41 (74.6) |
0.008 |
|
Type of atrial fibrillation, n (%) |
|
|
|
|
|
|
|
Paroxysmal |
48 (33.8) |
98 (49.0) |
0.01 |
22 (25.3) |
26 (47.3) |
0.008 |
|
Persistent |
24 (16.9) |
29 (14.50) |
0.01 |
14 (16.09) |
10 (18.2) |
0.008 |
|
Permanent |
70 (49.3) |
73 (36.5) |
0.01 |
51 (58.6) |
18 (32.7) |
0.006 |
|
Time interval since AF diagnosis, months |
36 (6-87.5) |
48 (12-120) |
0.3 |
48 (9.7-120) |
24 (3-60) |
0.2 |
|
Time interval since initiating the OAC, months |
10 (3-36) |
24 (7.7-72) |
0.0001 |
12 (4-48) |
5 (2-12) |
0.002 |
|
Comorbidities, n (%) |
|
|
|
|
|
|
|
Heart failure |
77 (53.5) |
91 (45.5) |
0.1 |
53 (60.2) |
23 (41.8) |
0.03 |
|
Arterial hypertension |
128 (88.9) |
164 (82.0) |
0.09 |
78 (88.6) |
49 (89.1) |
1.00 |
|
Diabetes mellitus |
46 (31.9) |
65 (32.7) |
0.0005 |
22 (15.3) |
24 (43.6) |
0.04 |
|
Valve replacement |
6 (4.2) |
17 (8.5) |
0.10 |
6 (6.8) |
0 (0.0) |
0.08 |
|
Mitral stenosis |
6 (4.2) |
4 (2.0) |
0.0005 |
6 (6.8) |
0 (0.0) |
0.002 |
|
Prior myocardial infarction |
38 (26.4) |
42 (21.0) |
0.2 |
15 (17.1) |
22 (40.0) |
0.003 |
|
Prior stroke |
16 (11.1) |
21 (10.5) |
0.8 |
7 (8.0) |
9 (16.7) |
0.1 |
|
Prior TIA |
4 (2.8) |
7 (3.5) |
0.7 |
2 (2.3) |
2 (3.6) |
0.6 |
|
Vascular disease |
17 (11.8) |
72 (36.0) |
0.001 |
7 (7.9) |
10 (18.2) |
0.1 |
|
History of major bleeding |
12 (8.3) |
11 (5.5) |
0.3 |
1 (1.1) |
4 (7.3) |
1.0 |
|
Easy bruising |
55 (38.2) |
76 (38.0) |
1.00 |
39 (44.3) |
39 (44.3) |
0.07 |
|
Gingival bleeding |
14 (9.7) |
26 (13.0) |
0.3 |
10 (11.4) |
10 (11.4) |
0.5 |
AF: atrial fibrillation, OAC: oral anticoagulation, TIA: transient ischaemic attack. Data are given as mean± standard deviation, median (interquartile range), or number (percentages).
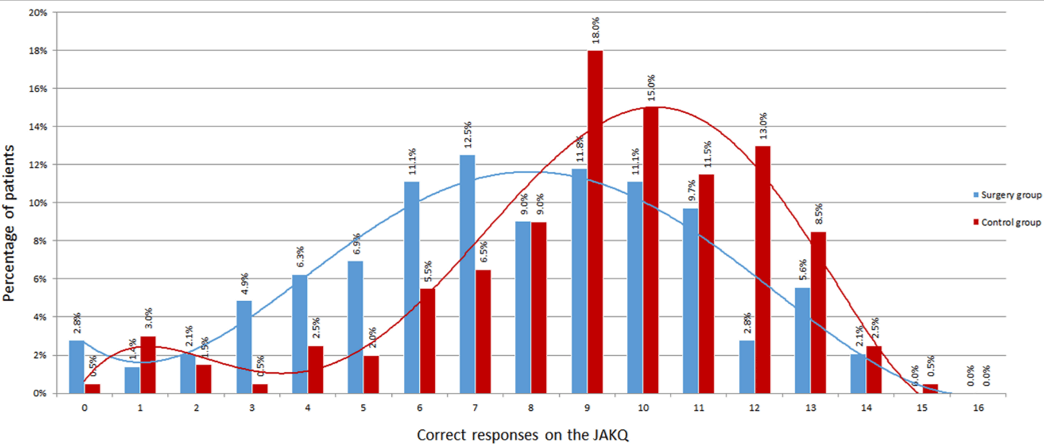
Figure 1: Distribution of correct responses in the surgery and control groups.
Table 2: Specific topics addressed in the JAKQ with percentage of correct responses among AF patients in surgery and control group.
|
|
Surgery group (n=144) |
Control Group (n=200) |
p value |
Valve surgery (n=88) |
Other surgery (n=55) |
p value |
|
8 questions about AF in general |
|
|
|
|
|
|
|
AF is a condition where the heart beats irregularly and often faster than normal |
96 (66.7) |
155 (77.5) |
0.02 |
62 (70.5) |
34 (61.8) |
0.3 |
|
AF is not always accompanied by symptoms |
26 (18.1) |
56 (28.0) |
0.03 |
14 (15.91) |
12 (21.8) |
0.3 |
|
Patients can detect AF by taking their pulse regularly |
53 (36.8) |
100 (50.0) |
0.01 |
34 (38.6) |
19 (34.6) |
0.7 |
|
AF can cause blood clots which can lead to stroke (cerebral infarction) |
78 (54.2) |
136 (68.3) |
0.009 |
45 (51.1) |
32 (58.2) |
0.4 |
|
Medication cannot prevent AF permanently, as the arrhythmia will increasingly occur with ageing, even when taking medication |
51 (35.7) |
63 (31.7) |
0.4 |
36 (40.9) |
15 (27.8) |
0.1 |
|
An AF patient should not go to the general practitioner or emergency room each time he/she feels AF |
25 (17.4) |
62 (31.2) |
0.003 |
13 (14.8) |
12 (21.8) |
0.3 |
|
Being overweight exacerbates AF |
71 (49.3) |
113 (57.1) |
0.1 |
43 (48.9) |
28 (50.9) |
0.8 |
|
Blood thinners are often prescribed for patients with AF in order to prevent the development of blood clots in the heart, which can lead to stroke |
87 (60.4) |
157 (78.9) |
0.0003 |
55 (62.5) |
32 (58.2) |
0.7 |
|
5 questions about OAC therapy |
|
|
|
|
|
|
|
Patients with AF should always take their blood thinners, even if they do not feel AF |
108 (75.0) |
167 (84.8) |
0.02 |
69 (78.4) |
38 (69.1) |
0.2 |
|
Possible side effects of blood thinners are the occurrence of bleedings and longer bleeding times in case of injuries |
64 (44.8) |
132 (67.0) |
0.0001 |
47 (53.4) |
16 (29.6) |
0.008 |
|
AF patients may only take painkillers based on paracetamol |
39 (27.5) |
84 (43.8) |
0.002 |
29 (33.3) |
10 (18.5) |
0.08 |
|
When AF patients regularly have minor nose bleeds (that spontaneously cease), they should contact the general practitioner or specialist, while continuing to take their blood thinner |
96 (67.1) |
139 (70.6) |
0.5 |
59 (67.8) |
36 (65.5) |
0.8 |
|
If an AF patient needs an operation, he/she should consult a doctor to discuss possible options |
76 (52.8) |
145 (73.6) |
0.0001 |
48 (54.6) |
27 (49.1) |
0.6 |
|
3 questions about VKA |
n=64 |
n=109 |
|
n=47 |
n=17 |
|
|
AF patients taking VKA should have their blood thinning checked at least once a month |
55 (85.9) |
96 (88.1) |
0.8 |
43 (91.5) |
12 (70.6) |
0.04 |
|
When AF patients taking VKA have forgotten to take their blood thinner, they should still take their forgotten pill (immediately or at the next dose) |
9 (14.1) |
29 (27.1) |
0.05 |
6 (12.8) |
3 (17.7) |
0.6 |
|
INR is a measure to check how thick or how thin the blood is |
40 (62.5) |
93 (86.9) |
0.0003 |
33 (70.2) |
7 (41.2) |
0.04 |
|
3 questions about NOAC |
n=96 |
n=101 |
|
n=49 |
n=46 |
|
|
For patients taking NOAC, it is important to take their blood thinner at the same time every day |
85 (88.5) |
93 (92.1) |
0.4 |
44 (89.8) |
40 (87.0) |
0.7 |
|
When AF patients taking NOAC have forgotten to take their blood thinner, they can still take that dose, unless the time till the next dose is less than the time after the missed dose |
26 (27.1) |
33 (32.4) |
0.4 |
16 (32.7) |
10 (21.7) |
0.2 |
|
The NOAC card should be shown to their general practitioner and specialist by AF patients |
22 (25.0) |
26 (36.1) |
0.1 |
10 (22.2) |
11 (26.2) |
0.8 |
INR: international normalized ratio, NOACs: non-vitamin K antagonist oral anticoagulants, VKA: vitamin K antagonist, other abbreviations- see (Table 1).
II General Results
Patients in the surgery group scored less on the JAKQ than in the control group (47±20 % vs. 59±18 %, p<0.001) (Figure 1). There were no questions in which the surgery group scored better. As shown in (Table 2), a low number of patients knew that AF is not always symptomatic in the surgery and control groups (18.1 vs. 28.0 %, p=0.03) or that they should not consult a physician every time they feel it (17.4 vs. 31.2%, respectively, p=0.003). Knowledge about possible use of painkillers was very low, with 27.5% in the surgery versus 43.8% in the control group with correct answers indicating paracetamol as a safe medication.
In the surgery group, a higher level of education was associated with better scores (mean score of patients with a primary education level: 42±18%; secondary education: 50±21% and higher education: 56±15%; p=0.009). Patients who took VKA in the past scored better in both groups (53% vs. 43% in the surgery group, p=0.001, and 62% vs 56% in the control group, p=0.01). The absence of heart failure predisposed to better scores in the surgery group (52% vs. 43%, p=0.005), but not in the control group. Patients with PAD scored fewer points in both groups (35% vs. 49%, p=0.008 and 55% vs. 61%, p=0.02, respectively). Patients with easy bruising had better scores in the surgery group (52% vs 44%, respectively, p=0.01). Interestingly, patients with prior valve replacement did not score better in either group of patients and when analyzing both groups together (p=0.1).
III Valvular Surgery Group Versus the Remaining Surgical Patients
There were 88 patients (61.5%) admitted for isolated valvular surgery and 26 patients (18.2%) admitted for CABG surgery. Seventeen patients were scheduled for valvular and CABG surgery (11.9%), and 12 were admitted for other cardiac surgery (8.4%). In one patient, the type of planned cardiac surgery was not noted. Groups of patients did not differ regarding age or type of AF (Supplementary Table 1). Patients scheduled for valvular surgery scored better than those scheduled for CABG surgery (49±19 vs. 35±18% respectively, p=0.002). The best results were achieved by patients admitted for other cardiac surgery, with a mean score of 56% (p=0.002). Valvular surgery group had a longer duration of AF (79.1 vs. 21.3 months, respectively, p=0.04). Among this group, current anticoagulant therapy was also administered for a longer period of time (34.0 vs. 7.7 months, p=0.01). The valvular group was also characterized by a higher percentage of patients who took VKA in the past (53.4% vs. 3.8%, p=0.005). VKA was the most common anticoagulant (44.3%) in the valvular group, while in the CABG group, NOACs were preferred (88.4%).
Regarding safety questions concerning possible painkiller administration and proceeding in case of a forgotten dose of VKA or NOAC, there were no differences between the two subgroups, and their level of knowledge was low (p=0.08; p=0.69; p=0.26, respectively). Of note, one-third of the valvular group (33.3%) and 8% of the CABG group knew that paracetamol-based painkillers are the safe option for pain relief, while the majority preferred widely available cyclooxygenase-1 inhibitors. Moreover, 12.8% of the valvular group knew what to do in case of a missed dose of VKA, and 70.2% of patients would wait till the next dose. Better results were scored by patients taking NOAC (32.6% in the valvular group and 16% in the CABG group) (Supplementary Table 2).
Discussion
The current study assessed the knowledge about the arrhythmia and its treatment among patients with AF admitted to the hospital for scheduled heart surgery as compared to the control patients hospitalized in our centre. We found a low level of knowledge on AF and OAC using the JAKQ and serious gaps, including the safety issues in anticoagulated patients both on VKA and NOAC. Our study provides evidence for the need for specific educational goals for this subgroup of the AF population given the perspective of long-term anticoagulation to prevent life-threatening thromboembolic events associated not only with chronic AF but also cardiac valve prosthesis implantation in a substantial proportion of surgical patients. This study shows that the JAKQ could also be used among AF patients scheduled for cardiac surgery.
The mean score in the current surgery group was 47±20% and was lower than in our controls (59±18%) and that reported by Desteghe et al. (56±19%) as well as by our group [18-20]. This difference cannot be explained by the patients’ age, which was lower in the surgery group compared with previous studies in AF patients [18, 19]. The surgery group was characterized by a shorter time interval since initiating the OAC compared with the control group. It is likely that with time their knowledge will increase. However, education of patients seems to be a key factor affecting knowledge of AF and anticoagulant therapy, because the current surgical patients had a lower level of education than those investigated by Desteghe et al. [18]. Hu et al. reported that patients 3 to 6 months after mechanical heart valve replacement with higher education levels had better warfarin knowledge scores [23]. The lower level of education has been reported to be associated with more frequent INR deviation from target values and inferior awareness of anticoagulation-related bleeding risk [15]. It is unclear whether educational programs aiming at improving the awareness of AF and its therapy can substantially enhance the knowledge in all patients but especially among individuals at a low level of general knowledge.
Given the fact that patients with artificial valve implantation require lifelong OAC to prevent thromboembolic events, knowledge gaps among VKA users reported by us are of particular importance [24, 25]. Reassuringly, we found that most of the surgical patients on VKA were aware of the mandatory monthly INR monitoring. However, the best results were achieved patients admitted for valvular surgery (91.5%), including a better knowledge about the definition of INR. These scores were higher compared with those reported by Desteghe et al. and in the EHRA study [15, 18]. Previous studies showed moderate or poor knowledge concerning the treatment among patients with artificial valve prostheses [23, 24, 26]. Improved knowledge has been associated with improved compliance and control [23]. This group must be educated particularly thoroughly using targeted educational programs linking two indications for chronic oral anticoagulation. Clarkesmith et al. recommended the educational intervention to be done every three to six months to maintain patient levels of adherence, the group who had the educational intervention spent statistically significant more time in the INR therapeutic range than the usual care group [27].
Appropriately informed and educated patients, who are also aware of threats arising from AF and advantages from a well-managed long-term treatment, would be more compliant and directly be willing to follow recommendations regarding anticoagulation therapy [11, 28]. Also, a patient-centered care strategy and a shared decision-making process can improve adherence and are underlined both in European and American Guidelines [11, 29]. This strategy may reduce therapy discontinuation, which occurs in up to 50% of patients per year [30]. Even patients who received oral and written explanations can have some knowledge gaps, which points out towards the need to develop comprehensive educational tools in which education is given in a more targeted and tailored manner using some new possibilities like smartphone applications, for example, developed by ESC [11, 14, 31]. Optimal education on anticoagulation for patients following cardiac surgery remains to be established.
The study has several limitations. The study was conducted at a tertiary high-volume hospital with a large cardiac surgery department; therefore, our results could not be easily translated to low-volume hospitals, but most likely, the level of knowledge would be even lower. The size of the study population was relatively small, but representative of real-life patients with AF referred for heart surgery. Further studies are needed to confirm the current findings. Education level was not assessed in the control group; therefore, this aspect could not be comparatively analyzed. The number of prior visits and contacts with the cardiologist in which there exists the opportunity to educate patients might impact the results; however, this factor was not assessed in this study. It is unclear whether appropriate education can improve the current results as well as if the low level of knowledge is associated with an increased risk of thromboembolism or bleeding events following surgery.
Conclusion
The present study demonstrates that among AF patients admitted for scheduled heart surgery, the level of knowledge of AF and anticoagulant treatment is lower compared to matched AF patients without any indication for heart surgery, including issues pertinent to the safety of long-term anticoagulation. These findings suggest that this patient group requires intensive educational efforts prior to elective cardiac surgery and following the procedure in order to improve the quality of postoperative care, including anticoagulation.
What is Already Known?
Unstable anticoagulation represents a major risk factor for thrombus formation after mechanical valve replacement.
What This Study Adds?
We identified specific knowledge gaps in this vulnerable patient subset in order to facilitate targeted educational efforts.
Conflicts of Interest
M. Konieczyńska took part in conferences sponsored by Bayer and Boehringer Ingelheim. J. Legutko received lecture honoraria from Bayer. A. Undas received lecture honoraria from Bayer, Boehringer Ingelheim, and Pfizer. The remaining authors declared no conflicts of interest.
Funding
This work was supported by the Jagiellonian University Medical College (K/ZDS/007717 to A.U.).
Article Info
Article Type
Research ArticlePublication history
Received: Mon 27, Jan 2020Accepted: Sat 15, Feb 2020
Published: Sat 29, Feb 2020
Copyright
© 2023 Anetta Undas . This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.01.07
Author Info
Aleksandra Lipska Anetta Undas Hein Heidbuchel Jacek Legutko Justyna Śliwińska Krzysztof Piotr Malinowski Lien Desteghe Mateusz Sitkowski Małgorzata Konieczyńska Tomasz Rajs
Corresponding Author
Anetta UndasInstitute of Cardiology, Jagiellonian University Medical College, Kraków, Poland
Figures & Tables
Table 1: Characteristics of the study population.
|
|
Surgery group (n=144) |
Control group (n=200) |
p-value |
Valve surgery (n=88) |
Other surgery (n=55) |
p-value |
|
Age, year |
68.9±8.4 |
68.4±11.7 |
0.6 |
68.8±9.0 |
68.9±7.3 |
0.9 |
|
Male gender, n (%) |
87 (60.4) |
118 (59.0) |
0.8 |
45 (51.1) |
41 (74.6) |
0.008 |
|
Type of atrial fibrillation, n (%) |
|
|
|
|
|
|
|
Paroxysmal |
48 (33.8) |
98 (49.0) |
0.01 |
22 (25.3) |
26 (47.3) |
0.008 |
|
Persistent |
24 (16.9) |
29 (14.50) |
0.01 |
14 (16.09) |
10 (18.2) |
0.008 |
|
Permanent |
70 (49.3) |
73 (36.5) |
0.01 |
51 (58.6) |
18 (32.7) |
0.006 |
|
Time interval since AF diagnosis, months |
36 (6-87.5) |
48 (12-120) |
0.3 |
48 (9.7-120) |
24 (3-60) |
0.2 |
|
Time interval since initiating the OAC, months |
10 (3-36) |
24 (7.7-72) |
0.0001 |
12 (4-48) |
5 (2-12) |
0.002 |
|
Comorbidities, n (%) |
|
|
|
|
|
|
|
Heart failure |
77 (53.5) |
91 (45.5) |
0.1 |
53 (60.2) |
23 (41.8) |
0.03 |
|
Arterial hypertension |
128 (88.9) |
164 (82.0) |
0.09 |
78 (88.6) |
49 (89.1) |
1.00 |
|
Diabetes mellitus |
46 (31.9) |
65 (32.7) |
0.0005 |
22 (15.3) |
24 (43.6) |
0.04 |
|
Valve replacement |
6 (4.2) |
17 (8.5) |
0.10 |
6 (6.8) |
0 (0.0) |
0.08 |
|
Mitral stenosis |
6 (4.2) |
4 (2.0) |
0.0005 |
6 (6.8) |
0 (0.0) |
0.002 |
|
Prior myocardial infarction |
38 (26.4) |
42 (21.0) |
0.2 |
15 (17.1) |
22 (40.0) |
0.003 |
|
Prior stroke |
16 (11.1) |
21 (10.5) |
0.8 |
7 (8.0) |
9 (16.7) |
0.1 |
|
Prior TIA |
4 (2.8) |
7 (3.5) |
0.7 |
2 (2.3) |
2 (3.6) |
0.6 |
|
Vascular disease |
17 (11.8) |
72 (36.0) |
0.001 |
7 (7.9) |
10 (18.2) |
0.1 |
|
History of major bleeding |
12 (8.3) |
11 (5.5) |
0.3 |
1 (1.1) |
4 (7.3) |
1.0 |
|
Easy bruising |
55 (38.2) |
76 (38.0) |
1.00 |
39 (44.3) |
39 (44.3) |
0.07 |
|
Gingival bleeding |
14 (9.7) |
26 (13.0) |
0.3 |
10 (11.4) |
10 (11.4) |
0.5 |
AF: atrial fibrillation, OAC: oral anticoagulation, TIA: transient ischaemic attack. Data are given as mean± standard deviation, median (interquartile range), or number (percentages).
Table 2: Specific topics addressed in the JAKQ with percentage of correct responses among AF patients in surgery and control group.
|
|
Surgery group (n=144) |
Control Group (n=200) |
p value |
Valve surgery (n=88) |
Other surgery (n=55) |
p value |
|
8 questions about AF in general |
|
|
|
|
|
|
|
AF is a condition where the heart beats irregularly and often faster than normal |
96 (66.7) |
155 (77.5) |
0.02 |
62 (70.5) |
34 (61.8) |
0.3 |
|
AF is not always accompanied by symptoms |
26 (18.1) |
56 (28.0) |
0.03 |
14 (15.91) |
12 (21.8) |
0.3 |
|
Patients can detect AF by taking their pulse regularly |
53 (36.8) |
100 (50.0) |
0.01 |
34 (38.6) |
19 (34.6) |
0.7 |
|
AF can cause blood clots which can lead to stroke (cerebral infarction) |
78 (54.2) |
136 (68.3) |
0.009 |
45 (51.1) |
32 (58.2) |
0.4 |
|
Medication cannot prevent AF permanently, as the arrhythmia will increasingly occur with ageing, even when taking medication |
51 (35.7) |
63 (31.7) |
0.4 |
36 (40.9) |
15 (27.8) |
0.1 |
|
An AF patient should not go to the general practitioner or emergency room each time he/she feels AF |
25 (17.4) |
62 (31.2) |
0.003 |
13 (14.8) |
12 (21.8) |
0.3 |
|
Being overweight exacerbates AF |
71 (49.3) |
113 (57.1) |
0.1 |
43 (48.9) |
28 (50.9) |
0.8 |
|
Blood thinners are often prescribed for patients with AF in order to prevent the development of blood clots in the heart, which can lead to stroke |
87 (60.4) |
157 (78.9) |
0.0003 |
55 (62.5) |
32 (58.2) |
0.7 |
|
5 questions about OAC therapy |
|
|
|
|
|
|
|
Patients with AF should always take their blood thinners, even if they do not feel AF |
108 (75.0) |
167 (84.8) |
0.02 |
69 (78.4) |
38 (69.1) |
0.2 |
|
Possible side effects of blood thinners are the occurrence of bleedings and longer bleeding times in case of injuries |
64 (44.8) |
132 (67.0) |
0.0001 |
47 (53.4) |
16 (29.6) |
0.008 |
|
AF patients may only take painkillers based on paracetamol |
39 (27.5) |
84 (43.8) |
0.002 |
29 (33.3) |
10 (18.5) |
0.08 |
|
When AF patients regularly have minor nose bleeds (that spontaneously cease), they should contact the general practitioner or specialist, while continuing to take their blood thinner |
96 (67.1) |
139 (70.6) |
0.5 |
59 (67.8) |
36 (65.5) |
0.8 |
|
If an AF patient needs an operation, he/she should consult a doctor to discuss possible options |
76 (52.8) |
145 (73.6) |
0.0001 |
48 (54.6) |
27 (49.1) |
0.6 |
|
3 questions about VKA |
n=64 |
n=109 |
|
n=47 |
n=17 |
|
|
AF patients taking VKA should have their blood thinning checked at least once a month |
55 (85.9) |
96 (88.1) |
0.8 |
43 (91.5) |
12 (70.6) |
0.04 |
|
When AF patients taking VKA have forgotten to take their blood thinner, they should still take their forgotten pill (immediately or at the next dose) |
9 (14.1) |
29 (27.1) |
0.05 |
6 (12.8) |
3 (17.7) |
0.6 |
|
INR is a measure to check how thick or how thin the blood is |
40 (62.5) |
93 (86.9) |
0.0003 |
33 (70.2) |
7 (41.2) |
0.04 |
|
3 questions about NOAC |
n=96 |
n=101 |
|
n=49 |
n=46 |
|
|
For patients taking NOAC, it is important to take their blood thinner at the same time every day |
85 (88.5) |
93 (92.1) |
0.4 |
44 (89.8) |
40 (87.0) |
0.7 |
|
When AF patients taking NOAC have forgotten to take their blood thinner, they can still take that dose, unless the time till the next dose is less than the time after the missed dose |
26 (27.1) |
33 (32.4) |
0.4 |
16 (32.7) |
10 (21.7) |
0.2 |
|
The NOAC card should be shown to their general practitioner and specialist by AF patients |
22 (25.0) |
26 (36.1) |
0.1 |
10 (22.2) |
11 (26.2) |
0.8 |
INR: international normalized ratio, NOACs: non-vitamin K antagonist oral anticoagulants, VKA: vitamin K antagonist, other abbreviations- see (Table 1).
References
- Stewart S, Hart CL, Hole DJ, McMurray JJ (2002) A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/ Paisley study. Am J Med 113: 359-364. [Crossref]
- Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988. [Crossref]
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE (1995) The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98: 476-484. [Crossref]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M et al. (2014) Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129: 837-847. [Crossref]
- Colilla S, Crow A, Petkun W, Singer DE, Simon T et al. (2013) Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 112: 1142-1147. [Crossref]
- Al Sarraf N, Thalib L, Hughes A, Tolan M, Young V et al. (2012) Effect of Preoperative Atrial Fibrillation on Postoperative Outcome following Cardiac Surgery. Cardiol Res Pract 2012: 272384. [Crossref]
- Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM (2011) A comparison of outcome in patients with preoperative atrial fibrillation and patients in sinus rhythm. Ann Thorac Surg 92: 1391-1395. [Crossref]
- Banach M, Mariscalco G, Ugurlucan M, Mikhailidis DP, Barylski M et al. (2008) The significance of preoperative atrial fibrillation in patients undergoing cardiac surgery: preoperative atrial fibrillation--still underestimated opponent. Europace 11: 1266-1270. [Crossref]
- Banach M, Goch A, Misztal M, Rysz J, Zaslonka J et al. (2008) Relation between postoperative mortality and atrial fibrillation before surgical revascularization--3-year follow-up. Thorac Cardiovasc Surg 56: 20-23. [Crossref]
- Ngaage DL, Schaff HV, Barnes SA, Sundt TM 3rd, Mullany CJ et al. (2006) Prognostic implications of preoperative atrial fibrillation in patients undergoing aortic valve replacement: is there an argument for concomitant arrhythmia surgery? Ann Thorac Surg 82: 1392-1399. [Crossref]
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37: 2893-2962. [Crossref]
- Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M et al. (2018) ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39: 1330-1393. [Crossref]
- Lin SS, Tiong LY, Asher CR, Murphy MT, Thomas JD et al. (2000) Prediction of thrombus-related mechanical prosthetic valve dysfunction using transesophageal echocardiography. Am J Cardiol 86: 1097-1101. [Crossref]
- Desteghe L, Engelhard L, Vijgen J, Koopman P, Dilling Boer D et al. (2019) Effect of reinforced, targeted in-person education using the Jessa Atrial fibrillation Knowledge Questionnaire in patients with atrial fibrillation: A randomized controlled trial. Eur J Cardiovasc Nurs 18: 194-203. [Crossref]
- Amara W, Larsen TB, Sciaraffia E, Hernandez Madrid A, Chen J et al. (2016) Patients' attitude and knowledge about oral anticoagulation therapy: results of a self-assessment survey in patients with atrial fibrillation conducted by the European Heart Rhythm Association. Europace 18: 151-155. [Crossref]
- Parys M, Kowalczuk Wieteska A, Kulik H, Majchrzyk I, Zembala M (2019) A novel survey examining the level of knowledge about anticoagulant and anti-infectious prophylaxis in patients after mechanical cardiac valve implantation. Kardiol Pol 77: 225-227. [Crossref]
- Tam W, Woo B, Lim TW (2019) Questionnaires designed to assess knowledge of atrial fibrillation. A systematic review. J Cardiovasc Nurs 34: E14-E21. [Crossref]
- Desteghe L, Engelhard L, Raymaekers Z, Kluts K, Vijgen J et al. (2016) Knowledge gaps in patients with atrial fibrillation revealed by a new validated knowledge questionnaire. Int J Cardiol 223: 906-914. [Crossref]
- Konieczyńska M, Sobieraj E, Bryk AH, Dębski M, Polak M et al. (2018) Differences in knowledge among patients with atrial fibrillation receiving non-vitamin K antagonist oral anticoagulants and vitamin K antagonists. Kardiol Pol 76: 1089-1096. [Crossref]
- Janion Sadowska A, Sadowski M, Konieczyńska M, Skonieczny G, Metzgier Gumiela A et al. (2019) Large regional differences in prescription patterns of oral anticoagulants and knowledge of the disease and therapy among Polish patients with atrial fibrillation. Kardiol Pol 77: 437-444. [Crossref]
- Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C et al. (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 52: 616-664. [Crossref]
- Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR et al. (2010) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 8: 202-204. [Crossref]
- Hu A, Chow CM, Dao D, Errett L, Keith M (2006) Factors influencing patient knowledge of warfain therapy after mechanical heart valve replacement. J Cardiovasc Nurs 21: 169-175. [Crossref]
- Sharaf AY, Abd El Fattah Ibrahim AF, Elhamami M (2017) Knowledge and adherence to oral anticoagulant therapy among patients with mechanical heart valve prosthesis. IOSR-JNHS 6: 19-29.
- Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R et al. (2007) Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28: 230-268. [Crossref]
- Rocha H, Rabelo E, Aliti G, de Souza E (2010) Knowledge of patients with mechanical valve prostheses concerning chronic oral anticoagulant therapy. Rev Latino Am Enfermagem 18: 696-702. [Crossref]
- Clarkesmith DE, Pattison HM, Lip GY, Lane DA (2013) Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomized trial. PLoS One 8: e74037. [Crossref]
- Rolls CA, Obamiro KO, Chalmers L, Bereznicki LRE (2017) The relationship between knowledge, health literacy, and adherence among patients taking oral anticoagulants for stroke thromboprophylaxis in atrial fibrillation. Cardiovasc Ther 35. [Crossref]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE et al. (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 64: 2305-2307. [Crossref]
- Noseworthy PA, Brito JP, Kunneman M, Hargraves IG, Zeballos Palacios C et al. (2019) Shared decision-making in atrial fibrillation: navigating complex issues in partnership with the patient. J Interv Card Electrophysiol 56: 159-163. [Crossref]
- Desteghe L, Germeys J, Vijgen J, Koopman P, Dilling Boer D et al. (2018) Effectiveness and usability of an online tailored education platform for atrial fibrillation patients undergoing a direct current cardioversion or pulmonary vein isolation. Int J Cardiol 272: 123-129. [Crossref]
