Evaluation of the Number, Biophysical and Multipotent Characteristics of Adipose Derived Stem Cells Harvested by SEFFI Procedure and Interaction with Different Type of Hyaluronic Acids
A B S T R A C T
Background: Injection of autologous adipose-derived stem cells (ADSCs) and a stromal vascular fraction (VSF) into dermal and subdermal layers promises regenerative advantages by improving skin volume and rejuvenation. Injectable hyaluronic acid (HA) is a temporary dermal filler that, by improving skin hydration, reduces the appearance of fine lines and wrinkles, facial folds and creates structure and volume to the face and lips. This study combined the grafting of micro fragmented fatty tissue with the hyaluronic acid filler procedure, using three different types of HA.
Methods: Each sample of micro fragmented adipose tissue harvested using the superficial enhanced fluid fat injection (SEFFI) technique collected from 8 patients were equally divided into two specimens. One of these (EMU specimens) was emulsified by gently applying ten back-and-forth passages from one syringe to another to fluidify the tissue. The other one was not emulsified (Ctrl/NON-EMU specimen). Both EMU and NON-EMU specimens were divided into four aliquots: one served as control, and the others were combined with each of three tested hyaluronic acids. Afterward, we assessed the cellularity of mesenchymal phenotype (defined as the number of adherent cells with mesenchymal phenotype per milliliter of adipose tissue) and the in vitro capacity of differentiation in mesenchymal lineages.
Results: Despite low cellularity from emulsified samples combined with HA, isolated cells could grow and expand in culture, thus proving their proliferative ability, showing “good quality” in all conditions (Ctrl/NON-EMU, EMU, and combined with HA). The cells could differentiate towards mesenchymal lineages, express mesenchymal markers by flow cytometry analysis, and maintain their stemness potential.
Conclusion: The combination of emulsified harvested tissue with HA products can be exploited to counteract the loss of volume and skin aging of the human face and body. This approach to regenerative aesthetic treatment is a promising treatment for facial antiaging therapy.
Keywords
Adipose-derived stem cells, autologous fat transfer, hyaluronic acid filler, stromal-vascular fraction, clinical regeneration applications
Introduction
Adipose tissue is a promising source of mesenchymal stem cells that share many characteristics with those extracted from bone marrow (MSCs) [1-4]. Adipose-derived mesenchymal stem cells (ADSCs) are isolated through enzymatical digestion from the stromal vascular fraction (SVF) that contains large numbers of different types of cells: adipocyte progenitors, pericytes, endothelial progenitor cells, and transit-amplifying cells [5]. ADSCs have been shown to possess the in vitro and in vivo potential to differentiate into different lineages like osteogenic, chondrogenic, myogenic, hepatogenic, and endothelial cells [6, 7]. Moreover, like all MSCs, they exhibit antifibrotic and immunomodulatory characteristics and improve fat graft survival by inducing angiogenesis [8-11]. Thanks to these characteristics, adipose tissue implantation has been used to enhance skin trophism, accelerate healing of chronic wounds and ulcers, and improve skin appearance after damage from radiotherapy [11-14]. The regenerative properties of the SVF and ADSCs have also found application in plastic, reconstructive and aesthetic surgery [15, 16]. This approach in regenerative therapy (RT), which exploits the antiaging effect of SFV cells and ADSCs, is a promising procedure and an answer to the increasing request of minimally invasive while effective, long-lasting, and safe treatments.
The Regenerative Therapy for Facial and Tissue Aging
There are two distinct skin aging processes: photoaging (extrinsic) and chronological (intrinsic) [17]. The characteristics of skin aging include wrinkles, dryness, laxity, thinning, irregular pigmentation, and loss of elasticity caused by environmental and genetic factors. Histology studies revealed that UVA exposure turns skin into a flattened dermo-epidermal interface, with loss of dermal papillae, with a consequent decrease in dermal thickness and vascularity. This phenomenon leads to decreased fibroblast activity and accidentally arranged elastin fibers [18]. Fibrous collagen is vital for skin strength and elasticity. The amount of this protein usually decreases with aging. Many studies in the aesthetic field for skin rejuvenation confirm that ADSCs can increase dermal collagen synthesis and even vascularity via the production of many cytokines and growth factors [15, 19-22]. Pieces of evidence on the potential differentiation of ADSCs into endothelial progenitor cells have been reported recently. ADSCs interact with endothelial cells and macrophages to increase monocyte chemoattracting protein-1 (MCP-1) and vascular endothelial growth factor (VEGF) secretion to regulate angiogenesis [23, 24].
It has been shown that ADSCs can secrete growth factors that influence fibroblast and keratinocyte proliferation, migration, specifically collagen synthesis, and other ECM proteins connected with tissue repair and regeneration [25, 26]. Some studies proved that ADSCs could improve and increase dermal thickness and reduce wrinkles, likely by inducing paracrine dermal fibrosis (DF) and angiogenesis [26-28]. Transforming Growth Factor Beta (TGF-β) appears to play a notable role in regulating skin mechanisms and preventing their impairment after injury or during aging. By targeting ADSCs and DF, TGF-β participates in skin regeneration by inducing skin cell proliferation, migration, enhancement of angiogenesis, regulating melanogenesis, and accelerating wound healing and closure [29]. Fibroblast Growth Factors as FGF-2 and b-FGF interact with the keratinocyte growth factor (KGF) to reduce wrinkles by targeting fibroblasts and keratinocytes, thus modulating, in the same way as the normal process of angiogenesis, the tissue regeneration during wound healing and significantly improving the antiaging process [30-32].
Approaching HA to Regenerative Medicine
On the other hand, over the last 15 years, hyaluronic acid (HA) has proven to be one of the most popular and safe injectable biomaterials for soft tissue correction: the notable growth popularity of the use of HA fillers to repair aging-related facial defects likely rely on their excellent safety profile, efficacy, and ease of administration [33]. HA is a non-sulfated glycosaminoglycan manly produced by mesenchymal cells. Although it can be found in any tissue of the organism, the skin is the richest one, with the HA content being approximately 50% of the total amount in the body. The roles of HA vary from joints hydration and lubrication to the modulation of inflammatory cells’ activation to enhance the immune response. Furthermore, it is a component of the extracellular matrix (ECM), the three-dimensional network fundamental for cells’ migration. Different manufacturing technologies allow the production of various HA fillers with different rheological properties due to differences in the HA concentration, three-dimensional links and structures, pore size distributions of the fibrous HA networks, and cohesivity/dispersivity ratio.
Recently, the combination of ADSCs and HA has been investigated for tissue engineering applications [34-36]. Within the biohybrid system, the biomaterial acts as a scaffold that mimics the three-dimensional structure of the tissue, generating a microenvironment closely like the ECM that promotes and assists the adhesion, proliferation, and differentiation process of stem cells [37-39]. This study wanted to assess the usefulness of combining the HA filler procedure with the grafting of micro fragmented adipose tissue rich in SVF and ADSCs in facial rejuvenation. We used three types of HA fillers that differed in HA concentration and crosslinked three-dimensional network structures. Since the main processes involved in facial and tissue aging are the loss of volume and skin aging, the combination of HA filler and stem cell therapy might open a new age of regenerative aesthetic treatment.
New technologies and techniques have been investigated to optimize fat tissue harvesting and reduce manipulation. In this study, we used the SEFFI (Superficial Enhanced Fluid Fat Injection) procedure. This technique was standardized by one of the authors of this study (AG) to obtain micro clusters of adipose tissue during the harvesting procedure while avoiding potentially harmful manipulations to fluidify the tissue [40-44]. The harvesting cannula used in the SEFFI technique is provided with multiples micro side portholes (0.8mm) that select micro clusters of adipose tissue. This tissue is fluid enough to be injected into the patient with no need for further manipulations, potentially harmful to cells, reducing viability and stemness in the adipose tissue.
Materials and Methods
I The Adipose Tissue Harvesting Procedure
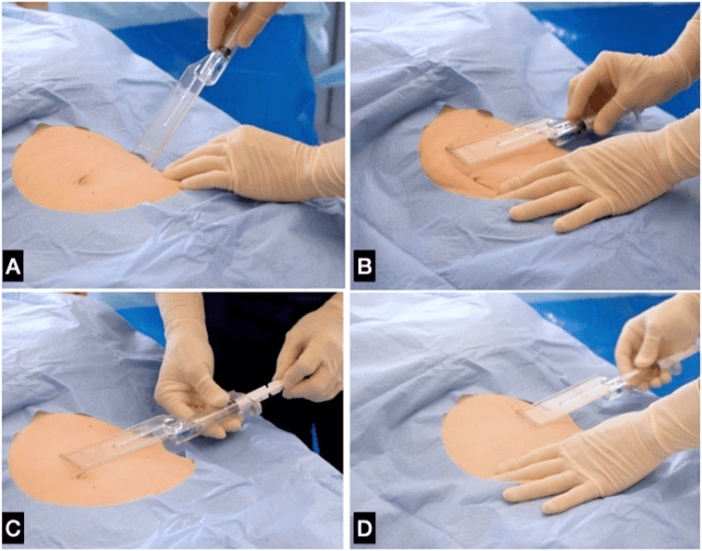
A total of 8 consecutive patients (6 females and two males) aged on average 47.7 years old were treated with the SEFFI technique to collect the fat tissue between February 2019 and March 2020. The SEFFI tissue harvesting technique has been previously described [42, 44]. Briefly, the procedure was performed under local anaesthesia using a 2-mm diameter microperforated cannula with 0.8 mm side portholes mounted inside the unique patented guide (Figure 1). The guide is addressed to standardize the procedure and guarantee that tunneling is performed in the subcutaneous tissue adjacent to the dermis, particularly rich in mesenchymal and vascular stem cells and at the same plane for all samples (i.e., 15 mm under the skin surface) [45, 46]. Both cannula and guide are included in the SEFFlLLER™ medical device (produced by SEFFILINE S.r.l. Bologna - Italy). Each harvested adipose tissue was gently washed, transferred into a sterile tube, and left in a vertical position for approximately 5 minutes to allow sedimentation and subsequent discard of the upper oily layer.
Figure 1: Manual aspiration/selection of the adipose tissue. A) The cannula is introduced perpendicular to the skin through a hole in the skin and B) positioned in the superficial subcutaneous plane; C) the plunger is locked in aspiration position and moved back and forth to harvest the fatty tissue clusters; D) the guide assembled with syringe and cannula. The cannula selects adipose tissue in small clusters without the need for substantial manipulation before injection. The unique guide allows for harvesting the fatty tissue in the superficial and standardized plain even if the physician has no specific liposuction skills; E) the micro fragmented adipose tissue is collected in the syringe.
II Cell Isolation and Combination with HA
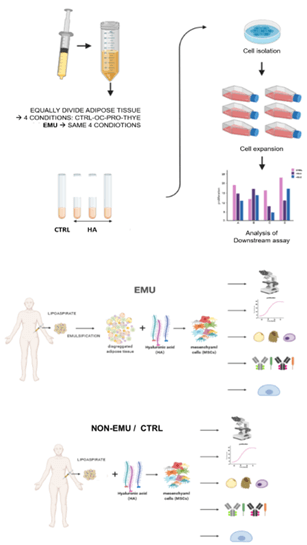
After removing the oily layer, each lipoaspirate sample was gently resuspended, and the volume was measured using a serological pipette. Then, the sample was measured for the cellularity (defined as the number of adherent cells with mesenchymal phenotype per milliliter of adipose tissue) and equally divided into two specimens. One of these (EMU specimens) was emulsified by gently applying ten back-and-forth passages from one syringe to another to fluidify the tissue (so that the injection can be performed using a small needle), and the other one was not emulsified (NON-EMU specimen). Both EMU and NON-EMU specimens were divided into four aliquots, of which one served as control and the other three were added with one of the hyaluronic acids as follows:
i. Ctrl – only adipose-derived stem cells.
ii. Ctrl+OC (OCLINE, produced by Officina Cosmetologica, Milano, Italy), which is non-cross-linked HA at a concentration of 2% (20mg/mL).
iii. Ctrl+PRO (Prophilo, produced by IBSA Pharmaceutical, Lugano, CH), a non-cross-linked HA at a concentration of 3.2% (32mg/mL), stabilized by hybrid complexes of acids of different molecular weight.
iv. Ctrl+THYE (STIMHA, produced by Documedica, Lugano, CH), a BDDE-cross-linked HA at a concentration of 2.5% (25 mg/mL).
The aim was to evaluate the combination of adipose stem cells /HA on the morphology, proliferation, and differentiation potential towards mesenchymal lineage (i.e., adipogenic, osteogenic, and chondrogenic lineages) and mesenchymal phenotype. The schematic representation of the experimental setup is reported in (Figure 2).
Figure 2: Schematic representation of the experimental setup.
III Feasibility Study and Cell Characterization
As a preliminary investigation, we used EMU and NON-EMU lipoaspirates samples obtained from two patients to optimize the isolation protocol. In this part of the study, we tested the combination of the stem cells with or without the hyaluronic acids OC and PRO. This part of the study aimed to investigate the harvesting and emulsification procedure as an innovative method to isolate adipose stem cells with no need for the enzymatic step. The HA-mix samples were prepared by adding each HA type with a ratio of [1:1] and gently resuspending the mixture to obtain a homogeneous solution. Aliquots of 500μL of tissue controls and HA-mix samples were plated in 10 cm2 of cell culture surface containing 3 mL of expansion medium composed of Dulbecco’s Modified Eagle’s Medium High Glucose (DMEM-H), 10% fetal bovine serum (FBS), and 1% Penicillin and Streptomycin (PS) (all materials were obtained from Lonza, Walkersville, MD, USA). The plating of lipoaspirate in culture plates was performed to mimic the in vivo surgery treatment. The adding of three types of HA was carried out to simulate the in vivo injection of HA in combination with adipose tissue and test in vitro their potential effect on cell isolation and properties.
After three days, the medium was replaced, leaving adipose tissue in the plate for another three days to allow cells to be released from the tissue and adhere to the plastic surface. Generally, after one week in culture first adherent cells are observed at the light microscope. Based on the results of the feasibility study, expanded cells were used for all the other evaluations. The medium was replaced, and the cells were let grow until they reached 70% of confluence. The attached cells were trypsinized and counted. Cellularity was calculated as the number of counted cells and divided by the milliliter of plated tissue. Cells were plated in a 96 well plate at a cell density of 3000 cells/cm2. The Alamar blue solution (AlamarBlue® Cell Viability Assay - Thermo Fisher Scientific, Waltham, MA, USA) was added to assess the proliferative potential of the adipose cells over time. This assay was performed according to the manufacturer’s instructions. Cells were monitored every day until cell confluence was reached. Fluorescence was read at a 530 nm to 560 nm excitation wavelength and a 590 nm emission wavelength using a multi-label plate reader Victor (PerkinElmer Wallace Inc., MA USA).
The day after plating, SA-β-galactosidase staining was performed to monitor the presence of senescent cells in culture using Senescence β-Galactosidase Staining Kit (9860S; Cell Signaling Technology, Inc., MA, USA) according to the manufacturer’s instructions. Based on the feasibility study results, expanded cells were also used to test the potential differentiation towards mesenchymal lineages. ADSCs were cultured in a growth medium to assess the adipogenic differentiation until they reached 90% confluence. Afterward, the medium was replaced with the adipogenic medium (StemPro Adipogenic Differentiation kit, Gibco). Cells were cultured for two weeks, and the medium was replaced every 3-4 days until the end of the treatment., Cells were fixed in 10% formalin for 10 minutes and stained with Oil Red O solution (Sigma-Aldrich, Merck Life Science, Milan, Italy) to visualize fat droplets. ADSCs were cultured in a growth medium until they reached 70% of confluence to evaluate osteogenic differentiation. The medium was replaced with the osteogenic induction medium (StemPro Osteogenesis Differentiation kit, Gibco) for 28 days. The medium was replaced every 3-4 days until the end of the differentiation. Then, matrix deposition was stained using Alizarin Red solution (40mM, pH 4.1, Sigma Aldrich).
To support chondrogenic differentiation, 100,000 cells were resuspended in 10 µL of PBS and plated for 1 hour at 37˚C in a humidified incubator. Pellet was cultured for one day in the growth medium, then replaced with the chondrogenic medium (StemPro Chondrogenesis Differentiation kit, Gibco). Differentiation assay lasted 21 days, with the medium being changed every 3-4 days. Micro-masses and differentiated adherent cells were visualized using Alcian blue staining using a light microscope. Each type of staining was quantified using the NIH ImageJ software to measure % of area stained. Expanded cells were also analysed for mesenchymal (CD105, CD90, and CD73), endothelial (CD31), and hematopoietic (CD34 and CD45) markers by flow cytometry.
IV Statistical Analysis
Cellularity data were plotted using Graph Pad software and used for statistical analysis using the Student’s t-test data, are presented as mean + standard deviation, as well as mean + standard error. A P-value less than 0.05 was considered statistically significant.
Results and Discussion
I Results of the Feasibility Study
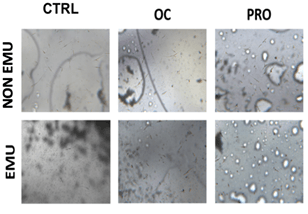
MSCs could be isolated from lipoaspirate samples in almost all conditions; furthermore, the cells showed mesenchymal morphology when observed at a light microscope (Figure 3). Cells were isolated for each condition (EMU and NON-EMU samples). Thus, from this preliminary study to test the assay’s feasibility, we could conclude that this type of isolation protocol could successfully recover mesenchymal-like cells. Besides, the comparison with the clinical procedure was maintained. Therefore, we proceeded with the other tests.
Figure 3: Cells were identified for each condition. Cell cultures were quite heterogeneous, with fibroblastic-like cells of different sizes and cells with long extrusions.
II Cellularity
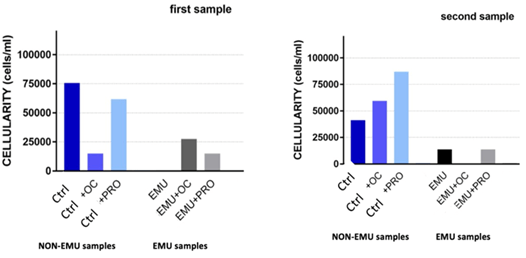
Table 1 summarizes the results on the cellularity obtained for each tested condition. Cell recovery from NON-EMU Ctrl samples was successful in 8 out of 8 total samples (100%), whereas the cell recovery from EMU Ctrl samples was successful in 3 cases out of 8 (37.5%). Some of the “void” cultures of EMU Ctrl samples were still void of cells when combined with all tested HAs. Cultures devoid of cells were also found in two NON-EMU Ctrl+PRO samples and one EMU Ctrl+OC sample. Only from one specimen of harvested adipose tissue were we able to derive cells were derived in all tested conditions. When data were combined and graphed to verify the effect of HA on cellularity (Figure 4), high variability between became visible samples probably due to biological variance, which is a well-known phenomenon in biology. NON-EMU samples showed high variability, as shown by the high standard deviation of each group (Figure 5, graph on the left). In contrast, EMU samples expressed a bit lower, yet notable, standard deviation. To better understand differences in cellularity among treatments, data were graphed with standard error mean (SEM, Figure 5, graph on the right). Cellularity slightly decreased in NON-EMU Ctrl+PRO and NON-EMU Ctrl+THYE samples, whereas no difference was noted among EMU samples incubated or not with HA.
Table 1: Cellularity (n. cells/ml) obtained from the cultures
of non-emulsified (Ctrl/NON-EMU) and emulsified (EMU) ADSCs specimens (collected
from the eight patients), with or without the addition of the three types of
HA.
|
|
Sample/patient number |
||||||||
|
Type of treatment |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
|
NON-EMU |
|
|
|
|
|
|
|
|
|
|
|
Ctrl |
75625 |
41250 |
37500 |
25000 |
461500 |
387500 |
13333 |
179166 |
|
|
Ctrl+OC |
15000 |
59583 |
12500 |
87500 |
435000 |
120000 |
52380 |
315476 |
|
|
Ctrl+PRO |
61875 |
87083 |
0 |
0 |
279000 |
105000 |
11904 |
208333 |
|
|
Ctrl+THYE |
n.a. |
n.a. |
12500 |
50000 |
420000 |
25000 |
4761 |
65476 |
|
EMU |
|
|
|
|
|
|
|
|
|
|
|
EMU |
0 |
13750 |
0 |
12500 |
0 |
0 |
0 |
216666 |
|
|
EMU+OC |
27500 |
0 |
0 |
112500 |
0 |
5000 |
0 |
184523 |
|
|
EMU+PRO |
15000 |
13750 |
0 |
25000 |
0 |
0 |
0 |
220238 |
|
|
EMU+THYE |
n.a. |
n.a. |
0 |
87500 |
0 |
10000 |
0 |
172619 |
n.a.: Sample Not Available.
Figure 4: Cellularity for the first two lipoaspirate samples. Cellularity is calculated by dividing the number of adherent cells per milliliters of adipose tissue plated.
Figure 5: Sample’s cellularity for each tested condition in non-emulsified (Ctrl) and emulsified (EMU) samples. Left graphs showed standard deviation (SD) data, while on the right side, data are represented with standard error mean (SEM).
III Proliferation
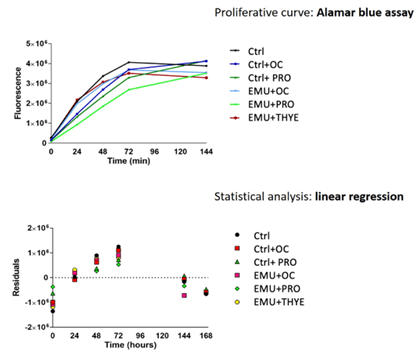
Even though the cellularity obtained from EMU samples combined with the tested HA was lower than the corresponding NON-EMU samples, cells could be expanded as shown by Alamar blue assay, thus proving their proliferative ability (Figure 6). Adipose-derived cells at the first passage in culture showed mesenchymal-like morphology; however, combining the different types of HA did not bring apparent differences. Cells from all samples were vital, in good conditions, and able to proliferate. The curves showed exponential growth, meaning that the cells were duplicating, and, after a couple of days, they reached the plateau due to cell culture confluence.
Figure 6: Sample’s proliferation by Alamar blue assay. Proliferative curves confirmed the duplication ability of adipose-derived cells in all conditions. The graphs below showed the linear regression analysis of samples, illustrating no statistical differences between conditions.
IV Senescent Phenotype
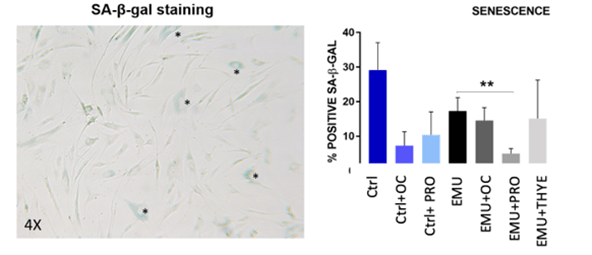
All cultured cells were stained for the senescence-associated β-galactosidase (SA-β-gal) to monitor the presence of senescent cells in culture. Cells, especially in vitro expanded cells, tend to stop proliferating, falling in a state of replicative senescence. A decrease in the number of SA-β-gal positive cells in NON-EMU Ctrl+OC and NON-EMU Ctrl+PRO cultures was observed. Overall, fewer senescent cells were found in all EMU cells combined with all tested HAs than in the EMU control cells (Figure 7). A statistically significant difference was found between the EMU Ctrl and the EMU Ctrl+PRO cultured cells.
Figure 7: Presence of senescent cells in culture. Senescence-associated β-galactosidase staining was performed on cells in culture. Blue-stained is the positive cells (signed with asterisks * in the representative figure on the left) and were counted compared to negative ones. The plot shows the expression of positive cells (**p<0.01, Student’s t-test).
V Adipogenic Differentiation
Overall, cells were able to differentiate towards adipogenic lineage in all conditions. With the only exception of the EMU Ctrl+THYE, all other EMU samples showed a slight increase towards adipogenic differentiation compared to NON-EMU ones (Figure 8). Moreover, the EMU Ctrl+PRO samples showed a better adipogenic differentiation than all the other HA combination samples. Oil droplets were visible in the cell cytoplasm, and it was also noted that few cells had smaller vacuoles, probably due to a lower grade of differentiation.
Figure 8: Adipogenic differentiation (**p<0.01, Student’s t-test).
VI Osteogenic Differentiation
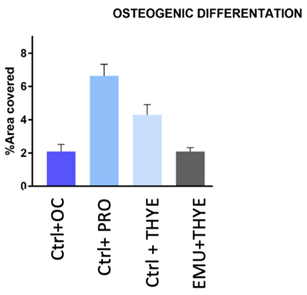
Cells were able to differentiate towards osteogenic lineage in all conditions analysed. Extracellular matrix was formed over cell layer, and it was stained in red by Alizarin staining. Among the tested samples, the NON-EMU Ctrl+PRO sample showed a better adipogenic differentiation than the other HA combination samples (Figure 9).
Figure 9: Osteogenic differentiation.
VII Chondrogenic Differentiation
The addition of each type of hyaluronic acid to NON-EMU cells improved differentiation, with the highest increase in NON-EMU Ctrl+THYE cells (Figure 10). EMU Ctrl+OC cells showed higher chondrogenic differentiation relative to EMU Ctrl ones, whereas the differentiation was lower in the EMU Ctrl+PRO cultured cells.
Figure 10: Chondrogenic differentiation (**p<0.01, *p<0.05, Student’s t-test).
VIII MSCs Phenotype
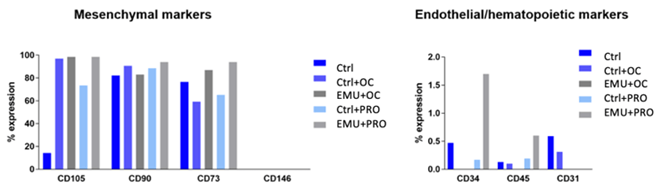
The analysis of the MSC phenotype could be conducted in one out of the eight collected specimens. We analysed the NON-EMU Ctrl, NON-EMU Ctrl+OC, NON-EMU Ctrl+PRO, EMU Ctrl+OC, and EMU Ctrl+PRO sample cells. Cells were negative for hematopoietic CD34 and CD45 and endothelial marker CD31. Expression was below 1% and was not affected by emulsification or the addition of HA. Mesenchymal expression in cells from NON-EMU Ctrl samples, emulsified and combined with hyaluronic acid, did not change drastically, except for CD105 expression. The addition of HA drastically improved CD105 expression (100%) in all conditions compared to NON-EMU Ctrl (14%). The NON-EMU Ctrl+PRO sample showed a lower expression compared to EMU Ctrl+PRO (73%). Interestingly, CD73 expression was lower in NON-EMU Ctrl cells combined with HA, whereas it increased when EMU Ctrl cells were added with OC and PRO hyaluronic acids (Figure 11).
Conclusion
Our study showed that it is possible to derive vital SVF cells and adipocytes from lipoaspirate samples harvested with SEFFI procedure with 0.8mm side portholes cannula using SEFFILLER® medical device. There was high variability in the cellularity among samples, and one of the causes could be donor biological variability. Although cellularity from emulsified samples combined with HA was lower, isolated cells could grow and expand in culture, thus proving their proliferative ability, and showed “good quality” in all conditions (NON-EMU, EMU, and combined with HA). Cells from tissue harvested with the SEFFI technique and emulsified are mesenchymal stem cells. They differentiate towards mesenchymal lineages, express mesenchymal markers by flow cytometry analysis, and maintain their stemness potential. Interestingly, the combination of hyaluronic acid in the harvested tissue and emulsified decreased the presence of senescent cells, especially for the mix with the PRO HA. Overall, the EMU samples show a slight increase towards adipogenic differentiation compared to Ctrl/NON-EMU ones. In particular, the Ctrl+PRO EMU+ PRO sample showed a better adipogenic differentiation compared to the samples combined with other HAs. The EMU +THYE cells showed a decrease in adipogenic differentiation ability.
Figure 11: MSCs phenotypes: MSCs phenotype. Mesenchymal expression in cells in Ctrl, EMU and combined with hyaluronic acid, did not change drastically, except for CD105 expression. The addition of HA drastically improved CD105 expression (100%) in all conditions compared to control (14%) and the combination with PRO showed a lower expression compared to the emulsified one (73%). Interestingly, CD73 expression was lower when cells were in combination with HA, but it increased when cells derived from the emulsification procedure and addition of the HA.
The addition of hyaluronic acid to tissue harvested with the SEFFI technique and emulsified or non-emulsified increased chondrogenic capacity, except for the EMU+PRO sample. This finding agrees with other papers showing HA’s ability to improve chondrogenic differentiation of adipose-derived stem cells [47]. Mesenchymal expression in emulsified cells combined with hyaluronic acid did not change drastically, except for CD105, whose expression was drastically improved by the addition of HA (100%). The CD73 expression was lower when non-emulsified cells were combined with HA but increased when emulsified cells were mixed with HA. Regenerative medicine is a high-potential sector of strategic developments in the medicine and health industry. The potential to make skin aging “curable” makes regenerative medicine of exceptionally crucial importance. The difficulty of ex vivo expansion and complexity of current Good Manufacturing Practice (cGMP) requirements for expanded cells lead to developing MSCs autologous strategies. Adipose tissue contains cells with phenotypic/transcription profiles of human mesenchymal stem cells (hMSCs) and pericytes. The availability of a minimally manipulated, hMSC/pericyte-enriched fat product would have remarkable biomedical and clinical relevance.
That’s why we aimed at developing a new fat tissue product, SEFFI + HA, available for the autologous ready-to-use treatment of human skin aging, which can be obtained without pre-treatment of enzymes and yields a highly homogeneous MSC population. The combination of SEFFI fresh tissue with HA product can be exploited to counteract the loss of volume and skin aging of the human face and body: examples of critical aesthetic areas of aging are the wrinkles around the mouth, loss of volume of the malar fat pad, tear trough periocular area, and hands and neck wrinkles. The non-expanded autologous fat product SEFFI with HA may pave the way to novel approaches and paradigms in facial rejuvenation within the context of personalized autologous regenerative medicine. Further studies involving more cases will be desirable, anyway the results of this study open the possibility to a new combined approach to facial rejuvenation treatments.
Conflicts of Interest
None.
Consent
All patients signed an informed consent.
Ethical Approval
The study was conducted following the tenets of the Declaration of Helsinki. Since the procedures were performed in one private center, international review board approval was not required.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 10, Sep 2021Accepted: Thu 07, Oct 2021
Published: Thu 21, Oct 2021
Copyright
© 2023 Alessandro Gennai. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2021.02.02
Author Info
Alessandro Gennai Bruno Bovani Mattia Colli Fabrizio Melfa Domenico Piccolo Rosalba Russo Matteo Tretti Clementoni Silvia Zia Barbara Roda Andrea Zattoni
Corresponding Author
Alessandro GennaiPlastic Surgeon, Private Practice, Studio Gennai, Bologna, Italy
Figures & Tables
Table 1: Cellularity (n. cells/ml) obtained from the cultures
of non-emulsified (Ctrl/NON-EMU) and emulsified (EMU) ADSCs specimens (collected
from the eight patients), with or without the addition of the three types of
HA.
|
|
Sample/patient number |
||||||||
|
Type of treatment |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
|
NON-EMU |
|
|
|
|
|
|
|
|
|
|
|
Ctrl |
75625 |
41250 |
37500 |
25000 |
461500 |
387500 |
13333 |
179166 |
|
|
Ctrl+OC |
15000 |
59583 |
12500 |
87500 |
435000 |
120000 |
52380 |
315476 |
|
|
Ctrl+PRO |
61875 |
87083 |
0 |
0 |
279000 |
105000 |
11904 |
208333 |
|
|
Ctrl+THYE |
n.a. |
n.a. |
12500 |
50000 |
420000 |
25000 |
4761 |
65476 |
|
EMU |
|
|
|
|
|
|
|
|
|
|
|
EMU |
0 |
13750 |
0 |
12500 |
0 |
0 |
0 |
216666 |
|
|
EMU+OC |
27500 |
0 |
0 |
112500 |
0 |
5000 |
0 |
184523 |
|
|
EMU+PRO |
15000 |
13750 |
0 |
25000 |
0 |
0 |
0 |
220238 |
|
|
EMU+THYE |
n.a. |
n.a. |
0 |
87500 |
0 |
10000 |
0 |
172619 |
n.a.: Sample Not Available.



References
1. Zuk PA, Zhu M,
Mizuno H, Huang J, Futrell JW et al. (2001) Multilineage cells from human
adipose tissue: implications for cell-based therapies. Tissue Eng 7:
211-228. [Crossref]
2. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI et al. (2002) Human
adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:
4279-4295. [Crossref]
3. Zuk PA (2010) The
adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell
21: 1783-1787. [Crossref]
4. Crisan M, Yap S,
Casteilla L, Chen CW, Corselli M et al. (2008) A perivascular origin for mesenchymal
stem cells in multiple human organs. Cell Stem Cell 3: 301-313. [Crossref]
5. Tallone T, Realini C, Böhmler A, Kornfeld C, Vassalli G et al. (2011) Adult human
adipose tissue contains several types of multipotent cells. J Cardiovasc
Transl Res 4: 200-210. [Crossref]
6. Huang JI, Beanes
SR, Zhu M, Lorenz HP, Hedrick MH et al. (2002) Rat extramedullary adipose
tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr
Surg 109: 1033-1041. [Crossref]
7. Fraser JK,
Schreiber R, Strem B, Zhu M, Alfonso Z et al. (2006) Plasticity of human
adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin
Pract Cardiovasc Med 3: S33-S37. [Crossref]
8. Caplan AI (2007)
Adult mesenchymal stem cells for tissue engineering versus regenerative
medicine. J Cell Physiol 213: 341-347. [Crossref]
9. Caplan AI, Dennis
JE (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem
98: 1076-1084. [Crossref]
10. Blaber SP, Webster
RA, Hill CJ, Breen EJ, Kuah D et al. (2012) Analysis of in vitro secretion
profiles from adipose-derived cell populations. J Transl Med 10: 172. [Crossref]
11. Coleman SR (2001)
Structural fat grafts: the ideal filler? Clin Plast Surg 28: 111-119. [Crossref]
12. Li K, Li F, Li J, Wang H, Zheng X et al. (2017) Increased survival of human
free fat grafts with varying densities of human adipose-derived stem cells and
platelet-rich plasma. J Tissue Eng Regen Med 11: 209-219. [Crossref]
13. Sultan SM, Barr JS,
Butala P, Davidson EH, Weinstein AL et al. (2012) Fat grafting accelerates
revascularization and decreases fibrosis following thermal injury. J Plast
Reconstr Aesthet Surg 65: 219-227. [Crossref]
14. Del Papa N, Di Luca G, Sambataro D, Zaccara E, Maglione W et al. (2015) Regional
implantation of autologous adipose tissue-derived cells induces a prompt
healing of long-lasting indolent digital ulcers in patients with systemic
sclerosis. Cell Transplant 24: 2297-2305. [Crossref]
15. Charles de Sá L,
Gontijo de Amorim NF, Takiya CM, Borojevic R, Benati D et al. (2015) Antiaging
treatment of the facial skin by fat graft and adipose-derived stem cells. Plast
Reconstr Surg 135: 999-1009. [Crossref]
16. Rigotti G, Charles
de Sá L, Gontijo de Amorim NF, Takiya CM, Amable PR et al. (2016) Expanded
Stem Cells, Stromal-Vascular Fraction, and Platelet-Rich Plasma Enriched Fat:
Comparing Results of Different Facial Rejuvenation Approaches in a Clinical
Trial. Aesthet Surg J 36: 261-270. [Crossref]
17. Fisher GJ, Kang S,
Varani J, Bata Csorgo Z, Wan Y et al. (2002) Mechanisms of photoaging and
chronological skin aging. Arch Dermatol 138: 1462-1470. [Crossref]
18. Rokhsar CK, Lee S,
Fitzpatrick RE (2005) Review of photorejuvenation: devices, cosmeceuticals, or
both? Dermatol Surg 31: 1166-1178. [Crossref]
19. Moon KC, Chung HY,
Han SK, Jeong SH, Dhong ES (2019) Possibility of Injecting Adipose-Derived
Stromal Vascular Fraction Cells to Accelerate Microcirculation in Ischemic
Diabetic Feet: A Pilot Study. Int J Stem Cells 12: 107-113. [Crossref]
20. Rubina K, Kalinina N, Efimenko A, Lopatina T, Melikhova V et al. (2009) Adipose
stromal cells stimulate angiogenesis via promoting progenitor cell
differentiation, secretion of angiogenic factors, and enhancing vessel
maturation. Tissue Eng Part A 15: 2039-2050. [Crossref]
21. Klar AS, Güven S,
Zimoch J, Zapiórkowska NA, Biedermann T et al. (2016) Characterization of
vasculogenic potential of human adipose-derived endothelial cells in a
three-dimensional vascularized skin substitute. Pediatr Surg Int 32:
17-27. [Crossref]
22. Bachmann S,
Jennewein M, Bubel M, Guthörl S, Pohlemann T et al. (2020) Interacting
adipose-derived stem cells and microvascular endothelial cells provide a
beneficial milieu for soft tissue healing. Mol Biol Rep 47: 111-122. [Crossref]
23. He Y, Xia J, Chen
H, Wang L, Deng C et al. (2019) Human adipose liquid extract induces angiogenesis
and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther
10: 252. [Crossref]
24. Na YK, Ban JJ, Lee
M, Im W, Kim M (2017) Wound healing potential of adipose tissue stem cell
extract. Biochem Biophys Res Commun 485: 30-34. [Crossref]
25. Hu L, Wang J, Zhou
X, Xiong Z, Zhao J et al. (2016) Exosomes derived from human adipose
mesenchymal stem cells accelerate cutaneous wound healing via optimizing the
characteristics of fibroblasts. Sci Rep 6: 32993. [Crossref]
26. Kim WS, Park BS,
Sung JH (2009) Protective role of adipose-derived stem cells and their soluble
factors in photoaging. Arch Dermatol Res 301: 329-336. [Crossref]
27. Zarei F, Abbaszadeh
A (2018) Stem cell and skin rejuvenation. J Cosmet Laser Ther 20:
193-197. [Crossref]
28. Acosta JC, Banito
A, Wuestefeld T, Georgilis A, Janich P et al. (2013) A complex secretory
program orchestrated by the inflammasome controls paracrine senescence. Nat
Cell Biol 15: 978-990. [Crossref]
29. Ehrlich M, Rao J,
Pabby A, Goldman MP (2006) Improvement in the appearance of wrinkles with
topical transforming growth factor beta (1) and l-ascorbic acid. Dermatol
Surg 32: 618-625. [Crossref]
30. Weiss RA, Weiss MA
(2014) Evaluation of a novel anti-aging topical formulation containing
cycloastragenol, growth factors, peptides, and antioxidants. J Drugs
Dermatol 13: 1135-1139. [Crossref]
31. Yang L, Zhang D, Wu
H, Xie S, Zhang M et al. (2018) Basic Fibroblast Growth Factor Influences
Epidermal Homeostasis of Living Skin Equivalents through Affecting Fibroblast
Phenotypes and Functions. Skin Pharmacol Physiol 31: 229-237. [Crossref]
32. Mazini L, Rochette
L, Malka G (2019) Growth Differentiation Factor 11 (GDF11)/Transforming Growth
Factor-β (TGF- β)/Mesenchymal Stem Cells (MSCs) Balance: A Complicated
Partnership in Skin Rejuvenation. J Embryol Stem Cell Res 3: 000122.
33. Molliard SG,
Bétemps JB, Hadjab B, Topchian D, Micheels P et al. (2018) Key rheological
properties of hyaluronic acid fillers: from tissue integration to product degradation.
Plast Aesthet Res 5: 17.
34. Poon CJ, Pereira E, Cotta MVPE, Sinha S, Palmer JA et al. (2013) Preparation
of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater
9: 5609-5620. [Crossref]
35. Altman AM, Khalek
FJA, Seidensticker M, Pinilla S, Yan Y et al. (2010) Human tissue-resident stem
cells combined with hyaluronic acid gel provide fibrovascular-integrated
soft-tissue augmentation in a murine photoaged skin model. Plast Reconstr
Surg 125: 63-73. [Crossref]
36. Argentati C, Morena F, Bazzucchi M, Armentano I, Emiliani C et al. (2018) Adipose Stem
Cell Translational Applications: From Bench-to-Bedside. Int J Mol Sci
19: 3475. [Crossref]
37. Martino S, D’Angelo F, Armentano I, Kenny JM, Orlacchio
A (2012) Stem cell-biomaterial interactions for regenerative medicine. Biotechnol
Adv 30: 338-351. [Crossref]
38. Murphy WL, McDevitt
TC, Engler AJ (2014) Materials as stem cell regulators. Nat Mater 13:
547-557. [Crossref]
39. Trappmann B,
Gautrot JE, Connelly JT, Strange DGT, Li Y et al. (2012) Extracellular-matrix
tethering regulates stem-cell fate. Nat Mater 11: 642-649. [Crossref]
40. Gennai A, Zia S, Roda B, Maggio A, Bonsi L et al. (2020) SEFFI
(Superficial Enhanced Fluid Fat Injection) for Aesthetic and Clinical
Regenerative Treatments. Global J Dermatol Venereol 8: 32-40.
41. Gennai A,
Bernardini FP (2017) Superficial enhanced fluid fat injection (SEFFI and
MicroSEFFI) in facial rejuvenation. CellR4 5: e223.
42. Gennai A, Zambelli A, Repaci E, Quarto R, Baldelli I et al. (2017) Skin
Rejuvenation and Volume Enhancement with the Micro Superficial Enhanced Fluid
Fat Injection (M-SEFFI) for Skin Aging of Periocular and Perioral Regions. Aesthet
Surg J 37: 14-23. [Crossref]
43. Bernardini FP,
Gennai A (2016) Fluid Fat Injection for Volume Restoration and Skin
Regeneration of the Periocular Aesthetic Unit. JAMA Facial Plast Surg
18: 68-70. [Crossref]
44. Bernardini FP, Gennai A, Izzo L, Zambelli A, Repaci E et al. (2015) Superficial
Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging
of the Face and Periocular Region. Aesthet Surg J 35: 504-515. [Crossref]
45. Clauser L, Lucchi
A, Tocco Tussardi T, Gardin C, Zavan B (2018) Autologous Fat Transfer for
Facial Augmentation and Regeneration: Role of Mesenchymal Stem Cells. Atlas
Oral Maxillofac Surg Clin North Am 26: 25-32. [Crossref]
46. Trivisonno A, Di Rocco G, Cannistra C, Finocchi V, Farr ST et al. (2014) Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J 34: 601-613. [Crossref]
47. Huang Y, Seitz D, König F, Müller PE, Jansson V et al. (2019) Induction of Articular Chondrogenesis by Chitosan/Hyaluronic-Acid-Based Biomimetic Matrices Using Human Adipose-Derived Stem Cells. Int J Mol Sci 20: 4487. [Crossref]
