Journals
Efficacy of a multicomponent nutraceutical on the normalization of liver functional parameters in patients with NAFLD: a double blind, randomized, clinical trial
A B S T R A C T
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in Western countries. The main preventive/therapeutic tool of NAFLD is currently the improvement of lifestyle. The purpose of this study is to evaluate the impact of a combined nutraceutical approach on the clinical and laboratory features of NAFLD patients. We consecutively enrolled 80 NAFLD patients in a double-blind, placebo-controlled, randomized clinical trial. After a 4 weeks stabilization diet period, we randomized them to assume a combined multivitamin/multimineral/botanical nutraceutical (MetaclearTM) 2 tablets per day or an identical placebo for 3 months. Patients were rechecked after further 30 days. Liver parameters significantly improved in the combined nutraceutical treated subjects. In particular, in a generalized linear mixed model with Fatty Liver Index (FLI) as dependent variable a significant period by treatment effect was observed (F-value = 22.5, p < 0.001). For the patients treated with the combined nutraceutical, FLI decreased with on average 11.9 units and 13.6 units at the end of treatment and the follow-up visit compared to baseline, respectively. The decrease in FLI from baseline to the end of treatment and to follow-up was on average 5.9 units higher (95%C 3.3-8.4, p < 0.001) and 8.6 units higher (95%CI 6.0-11.1, p < 0.001) for the combined nutraceutical treated group in comparison to the placebo group, respectively. The short-term treatment with the combined nutraceutical has been associated with a significant improvement of NAFLD biomarkers.
K E Y W O R D S
NAFLD,fatty liver, nutraceuticals,herbal supplements,dietary supplements,clinical trial
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a potentially evolutive condition characterized by a progressive infiltration of fat in the hepatocytes and comprises a wide spectrum of hepatic disorders that are not linked to other pathological conditions, such as viral hepatitis, alcohol consumption (>20 gr/day) and chronic drugs use [ ]. In the last years, the incidence of NAFLD has shown an exponential increase in western countries. The prevalence of NAFLD in developed countries is estimated to be about 30% of adult population, 15% of children, and more than 50% of overweight, obese, and type 2 diabetics [2]. However, also in Asia the disease is becoming increasingly common, with a prevalence up to 15% reported in China [3].
The clinically aggressive and irreversible variant of NAFLD, non-alcoholic steatohepatitis (NASH), affects about 5% of the general adult population, and 20% of obese people [4]. It’s characterized by inflammation and progressive tissue degeneration of the liver parenchyma: in general, about 1/3 of the cases of NAFLD tend to become NASH, and 15% of these can degenerate into cirrhosis. In addition, NAFLD is itself a risk factor for the development of cardiovascular disease and type 2 diabetes, and preliminary data suggest that it may also be associated with a greater incidence of hepatic and extra-hepatic cancers [5-7]. A meta-analysis of six studies (25,837 people, of whom 5953 were affected by NAFLD) showed that patients with NAFLD had a relative risk of total CV events of 1.77 (95% CI 1.26–2.48, p< 0.001); the relative risk increased to 2.26 (95% CI: 1.04–4.92, p < 0.001) with regard to coronary artery disease and to 2.09 (95% CI: 1.46–2.98, p < 0.001) relative to ischemic stroke. Furthermore, the presence of NAFLD seems to significantly increase the relative risk of mortality due to cardiovascular causes (RR 1.46, 95% CI 1.31–1.64, p < 0.001) [8].
On the other hand, overweight/obesity, insulin resistance/type 2 diabetes, hypertriglyceridaemia and the related dietary-behavioural triggers the high intake of beverages as well sweetened with fructose are the main risk factors for NAFLD [9]. As reported by observational studies, consumption of sugared soft drinks per se increases the risk of developing NAFLD of around 55% [10]. Since NAFLD pathogenesis involves both genetic, epigenetic and environmental factors, it is widely accepted that the disease can be considered a multifactorial pathology [11]. Even if NAFLD is often related to poor lifestyle habits, among the emerging risk factors there are the smoking habit and the Obstructive Sleep Apnoea Syndrome (OSAS), but also insomnia and excessive daytime sleepiness unrelated to nocturnal sleep apnoea [12, 13]. Finally, also the strong association between hypothyroidism and NAFLD has recently been confirmed by a meta-analysis of 13 prospective studies [14].
Treatment of NAFLD remains difficult pending specific drug therapies. The cornerstone of treatment is achieving weight control and reduction in cardiovascular risk factors. Currently the most effective treatment is weight loss by a combination of dietary modifications and exercise in order to reduce insulin resistance [15, 16]. Since NAFLD has many risk factors similar to the ones for CVD, the general indications are the same, including a relatively low-calorie diet (caloric quantity proportional to energy consumption), with carbohydrates predominantly with a low glycemic index and minimizing the consumption of fructose, alcohol, saturated and trans-unsaturated fats [17, 18]. The adherence to Mediterranean Diet seems to be an important predictor of liver fat content in people with NAFLD and also the coffee compsumption is related to a lower risk of fibrosis as summarised in a recent metanalysis regarding coffee drinkers patients with NAFLD [19, 20]. At the same time, physical activity is fundamental: a recent meta-analysis shows that the difference in risk of developing NAFLD among sedentary and physically is 21% in observational studies and 57% in case-control studies [21]. On the other hand, regardless of diet, the greater the frequency and intensity of physical activity, the greater the reduction of transaminase levels and the degree of hepatosteatosis, especially in overweight subjects [22].
Then, some nutraceuticals seem to improve NAFLD, when prescribed on top of therapeutic lifestyle changes [23]. For instance, Silymarin, a mixture of flavolignans extracted from Sylibum marianum, acting through different mechanisms and complex biological interactions has largely shown to improve the prognosis of patients affected by chronic liver diseases [24].The actions of berberine, curcumin and coenzyme q10 are related to the improvement of levels of indirect markers of hepatosteatosis (Hepatic Steatosis Index, Lipid Accumulation Product) for short-term supplements (2–4 months), even if each of these nutraceuticals showed any bioavailability problems [25-27]. In accordance with the parallel-hit hypothesis, stating that the development of liver disease involves a group of simultaneous alterations in the organism, combinations of different bioactive compounds that act against complementary targets have been examined [28]. Thus, most probably a combination of different nutritional interventions is needed to tackle the pathology [29].
The objective of the study is to examine whether patients with metabolic syndrome and NAFLD show reduced or normalized levels of hepatic functional parameters after taking a nutraceutical based on vitamins, minerals, amino acids and specific herbals.
Materials and Methods
Study design and study participants
This parallel arm, single-blind, placebo-controlled, randomized clinical trial was carried out in 80 adult subjects affected by metabolic syndrome and NAFLD, pharmacologically untreated, in primary prevention for cardiovascular diseases, consecutively enrolled in the ambulatory service of cardiovascular disease prevention in the Medical and Surgical Sciences Department of the University of Bologna. Metabolic syndrome was identified based on the ATPIII/IDF criteria [30]. The components were defined using the following ATPIII categorizations: 1) high blood pressure (BP ≥130/85 mmHg); 2) hypertriglyceridemia (TG ≥150 mg/dl), 3) low HDL-C (< 40 mg/dl for men and < 50 mg/dl for women); 4) hyperglycaemia (100 mg/dl >FPG), 5) waist circumference >102 cm for men and >88 cm for women (suggestive of high abdominal obesity). Subjects with at least three of the previous components were classified as having Metabolic syndrome. NAFLD was identified as having Fatty Liver Index (FLI) >60 or fatty liver confirmed by ultrasounds.
Exclusion criteria were:
- GOT, GPT or gGT >3 times ULN
- Alcoholism in active phase
- Diabetes mellitus with pharmacologic treatment
- Severe obesity (Body mass index >35 kg/m2)
- Inflammatory bowel disease
- Known individual intolerance to the components of the tested product
The study was fully conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients before the inclusion in the study.
At baseline, patients were given standard behavioral and qualitative (not quantitative) dietary suggestions to correct unhealthy habits. Standard diet advice was given by a dietitian and/or specialist doctor. Dietitian and/or specialist doctor periodically provided instruction on dietary intake recording procedures as part of a behavior modification program and then later used the subject’s food diaries for counseling. In particular subjects were instructed to follow general indication of a Mediterranean diet, avoiding excessive intake of dairy products and red meat derived products during the study, maintaining overall constant dietary habits. Individuals were also generically encouraged to increase their physical activity by walking briskly for 20 to 30 minutes, 3 to 5 times per week, or by cycling.
After 28 days, the subjects were randomized to be treated with a combined nutraceutical 2 tabs per day (MetaclearTM, kindly provided by Metagenics Europe Ltd., Belgium) or Placebo 2 tabs per day for three months. The composition of the tested product is detailed in (Table 1).
Table 1: Description of the tested product
|
%RI* |
|||
|
Vitamin A (retinyl palmitate) |
125 µg |
16% |
|
|
Vitamin B1 (thiamine monohydrate) |
2,1 mg |
191% |
|
|
Vitamin B2 (riboflavin) |
2,4 mg |
171% |
|
|
Vitamin B3 (niacinamide, niacin) |
11,7 mg |
73% |
|
|
Vitamine B5 (calcium pantothenate) |
9,0 mg |
150% |
|
|
Vitamine B6 (pyridoxal-5-phosphate) |
3,0 mg |
214% |
|
|
Vitamine B12 (methylcobalamine) |
25 µg |
100% |
|
|
Vitamine C (magnesium ascorbate) |
66,67 mg |
83% |
|
|
Vitamine D (cholecalcipherol) |
25 µg |
500% |
|
|
Vitamine E (D-alpha tocopheryl acetate) |
17,01 mg |
142% |
|
|
Biotin |
33,30 µg |
67% |
|
|
Folate (calcium-L-methylfolate) |
134 µg |
67% |
|
|
Copper (copper citrate) |
333 µg |
33% |
|
|
Manganese (manganese citrate) |
0,83 mg |
42% |
|
|
Molybdenum (sodium molybdate) |
33,3 µg |
67% |
|
|
Selenium (selenium methionine) |
25 µg |
45% |
|
|
Zinc (zinc citrate) |
3.3 mg |
33% |
|
|
Artichoke plant extract (Cynara scolymus) |
75 mg |
||
|
Contains cynarine |
3,75 mg |
||
|
Green tea leaf extract (Camellia sinensis) |
50 mg |
||
|
Contains: polyphenols |
Min 25 mg |
||
|
Catechins |
10-15 mg |
||
|
epigallocatechin-3-gallate |
3,5-6,5 mg |
||
|
Caffein |
2-3 mg |
||
|
Milk thistle seed extract (Silybum marianum) |
100 mg |
||
|
Contains silymarine |
80 mg |
||
|
Pomegranateextract (Punica granatum) |
100 mg |
||
|
Contains ellagic acid |
40 mg |
||
|
Watercress leaf extract (Nasturtium officinale) |
100 mg |
||
|
N-acetyl-L-cysteine |
150 mg |
||
|
Choline |
82,5 mg |
||
|
Alpha-lipoic acid |
50 mg |
||
|
L-cysteine HCl |
5 mg |
||
|
L-glutathione |
10 mg |
||
|
L-glycine |
400 mg |
||
|
L-lysine HCl |
35 mg |
||
|
L-threonine |
35 mg |
||
|
Potassium citrate |
214 mg |
||
|
Taurin |
100 mg |
||
|
Sodium sulfate |
50 mg |
||
|
*RI = Reference Intake |
|||
Clinical and laboratory data have been obtained at the baseline, after three months, and four weeks after the last drug assumption (follow-up visit) (Figure 1).
Figure 1: Study design
Randomization was done using a drawing of envelopes containing randomization codes prepared by an independent statistician and specific software. The envelopes were then further mixed and distributed to the investigators who assigned the randomization code in a progressive way to the enrolled subjects. A copy of the code was provided only to the person responsible of performing the statistical analysis. Throughout the study, we instructed patients to take the new product first dose on the day after they were given the study product in a blinded box. At the same time, all unused products were retrieved for inventory. Product compliance was assessed by counting the number of product doses returned at the time of specified clinic visits. At the end of the study, patients were asked to evaluate the treatment acceptability in term of “very low”, “low”, “good”, or “very good”.
Assessments
Patients’ personal history was evaluated, paying attention to cardiovascular disease and other diseases, dietary and smoking habits, physical activity, and pharmacological treatment. At each visit body weight and waist circumference were collected. Body mass index (BMI) was calculated as weight in kilograms (kg) divided by height in meters squared (m2). Height and weight were measured by standard procedures to the nearest 0.1 cm and 0.1 kg respectively, with subjects standing erect with eyes directed straight ahead, wearing light clothes, and with bare feet. Waist circumference (WC) was measured at the end of a normal expiration, in a horizontal plane at the midpoint between the inferior margin of the last rib and the superior iliac crest. Office blood pressure was also measured by standardized methodology [31].
All plasma parameters were obtained after a 12-hour overnight fast. Venous blood samples were drawn by a nurse in all patients between 8:00 a.m. and 9:00 a.m. Plasma used was obtained by addition of Na2EDTA (1 mg=mL) and centrifuged at 3,000 g for 15 minutes at 48°C. Immediately after centrifugation plasma samples were frozen and stored at -80°C for no more than 3 months. The following parameters were evaluated via standardized methods: total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), triglycerides (TG), LDL-Cholesterol (LDL-C), glucose, liver transaminases, and gamma-Glutamyl Transferase (gamma-GT) TC and LDL-C were measured at the baseline only [32].
Lipid accumulation product (LAP) was calculated as (WC − 65) × TG (expressed in mmol/L) for men and (WC − 58) × TG (expressed in mmol/L) for women, and hepatic steatosis index (HSI) resulted from 8 × ALT/AST ratio + BMI (+2 for women) [33, 34]. FLI was calculated as follows: [e0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745/(1 + e0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745)] × 100 [35].
Statistical Analysis
The parameters to assess efficacy of the tested combined nutraceutical vs. placebo were the change in FPG, AST, ALT, gamma-GT and HSI, after 84 (end of treatment) and 114 (follow-up) days, compared to baseline. Efficacy endpoints were analysed for the ITT population. Continuous data were summarized by their mean, standard deviation (SD), median, minimum and maximum. Categorical data were summarized by frequencies and percentages. Mean absolute changes in plasma levels of FPG, AST, ALT and gamma-GT and the index HSI from baseline (T0) at the end of treatment (T84) and at follow-up (T114) between the tested combined nutraceutical and placebo were evaluated with linear mixed-effects models, using the lmne package in the R environment [36, 37]. Patients were specified as a random factor (i.e. random intercept model). The assumptions underlying the analysis were verified using diagnostic plots (QQ-plots for the normality of the error term). All descriptive and statistical analyses were performed in R version 3.3.1. (R development core team, 2017). A p-value < 0.05 was considered as statistically significant. No imputation of missing data is performed. The amount of missing data is presented in the tables wherever appropriate.
Results
All patients completed the trial: no patient dropped out from the study because of adverse events. One subject treated with the combined nutraceutical and one treated with placebo claimed mild and transient abdominal discomfort. Compliance was high, being 92% in both treatment groups. The overall acceptability of the proposed treatment was defined as good-very good by the 90% of the nutraceutical treated patients and by the 86% of the placebo treated ones. At the baseline, enrolled patients were age- and sex- matched (M: 65% in the placebo group, 72.5% in the combined nutraceutical one). The baseline characteristics of patients assigned to the different treatments were similar, and no significant differences were observed regarding the studied parameters (Table 2). Liver parameters are reported in (Table 3).
From the randomization visit to the end of the study, the enrolled subjects maintained overall a similar dietary pattern, without significant change in total energy, total cholesterol and total saturated fatty acid intake. Mean waist circumference and BMI were very similar at baseline, the end of treatment and follow-up period for both the combined nutraceutical and the placebo treated group. In a generalized linear mixed model with TG as dependent variable, period (F-value= 35.3, p< 0.001) was significant but there was no evidence of a treatment effect (F-value= 0.08, p= 0.775). TG reduced with on average 36.9 units and 42.7 units at the end of treatment and at the follow-up visit compared to baseline, respectively. At the same time, in a generalized linear mixed model with HDL as dependent variable, period (F-value= 48.2, p < 0.001) was significant but there was no evidence of a treatment effect (F-value= 0.95, p= 0.334). HDL increased on average 2.5 units and 2.1 units at the end of treatment and at the follow-up visit compared to baseline. FPG reduced with on average 3.4 units and 4.2 units at the end of treatment and at the follow-up visit compared to baseline, respectively, but there was no evidence of a treatment effect (F-value = 2.9, p = 0.093).
For the patients in the combined nutraceutical, AST reduced with on average 13.2 units and 12.4 units at the end of treatment and at the follow-up visit compared to baseline, respectively. For the patients in the placebo group, AST reduced with on average 3.5 units and 0.3 units at the end of treatment and at the follow-up visit compared to baseline, respectively. This difference in reduction between the treatments was significant. The reduction in AST from baseline at the end of treatment and at follow-up was on average 9.7 units higher (95% CI: 6.7 - 12.8, p < 0.001) and 12.1 units higher (95% CI: 9.0 - 15.2, p < 0.001) for the combined nutraceutical group in comparison to the placebo group, respectively. This is also illustrated in the plots correspond with individual changes from baseline at end of treatment (T84) and at follow-up (T114) (figure 2). ALT reduced with on average 9.0 units and 9.2 units at the end of treatment and at the follow-up visit compared to baseline, respectively, even if there was no evidence of a treatment effect (F-value = 1.2, p = 0.271).
Table 2: Patient characteristics at baseline (data reported as mean and standard deviation)
|
|
Placebo |
Combined nutraceutical |
|
Placebo |
Combined nutraceutical |
|
Age (years) |
57.4 (13.6) |
59.6 (12.5) |
Total Cholesterol (mg/dL) |
224.4 (38.5) |
221.3 (34.8) |
|
Systolic blood pressure (mmHg) |
143.3 (20.4) |
141.5 (17.9) |
Triglycerides (mg/dL) |
189.1 (98) |
199.3 (152) |
|
Diastolic blood pressure (mmHg) |
78 (9.4) |
75 (7.7) |
HDL-Cholesterol(mg/dL) |
42.5 (11.5) |
39.4 (11.9) |
|
Waist circumference (cm) |
103.4 (7.6) |
100.5 (5.9) |
LDL-Cholesterol (mg/dL) |
143.8 (33.9) |
148.2 (34.9) |
|
Body Mass Index (kg/m2) |
29.8 (2.9) |
28.9 (2.4) |
Fasting Plasma Glucose (mg/dL) |
97.7 (11.6) |
95.3 (11.8) |
Table 3: Liver parameters at the baseline in both studied groups (data reported as mean and standard deviation)
|
|
Placebo |
Combined nutraceutical |
|
Placebo |
Combined nutraceutical |
|
Aspartate transaminase (AST) |
39.2 (6.9) |
39.8 (7.2) |
Fatty Liver Index (FLI) |
87.5 (11.1) |
88 (8.9) |
|
Alanine transaminase (ALT) |
41.4 (9.7)
|
41.9 (10.3)
|
Hepatic Steatosis Index (HIS) |
31.6 (2.8) |
30.6 (3.1) |
|
gamma-Glutamyl Transferase |
44.2(13.6) |
44.9 (12.9)
|
Lipid Accumulation Product (LAP) |
84.1 (43.2) |
78.5 (50.1) |
For the patients in the combined nutraceutical, gamma-GT reduced with on average 14.6 units and 15.9 units at the end of treatment and at the follow-up visit compared to baseline, respectively. For the patients in the placebo group, gammaGT reduced with on average 9.3 units and 7.5 units at the end of treatment and at the follow-up visit compared to baseline, respectively. This difference in reduction between the treatments was significant. The reduction in gamma-GT from baseline at the end of treatment and at follow-up was on average 5.4 units higher (95% CI: 0.5 - 10.2, p= 0.030) and 8.4 units higher (95% CI: 3.6 - 13.2, p= 0.001) for the combined nutraceutical group in comparison to the placebo group, respectively. This is also illustrated in the plots correspond with individual changes from baseline at end of treatment (T84) and at follow-up (T114) (Figure 3). In the active group, FLI decreased with on average 11.9 units and 13.6 units at the end of treatment and at the follow-up visit compared to baseline, respectively. For the patients in the placebo group, FLI decreased with on average 6.0 units and 5.1 units at the end of treatment and at the follow-up visit compared to baseline, respectively. This difference in decrease between the treatments was significant. The decrease in FLI from baseline at the end of treatment and at follow-up was on average 5.9 units higher (95% CI: 3.3 - 8.4, p < 0.001) and 8.6 units higher (95%CI: 6.0 - 11.1, p < 0.001) for the combined nutraceutical group in comparison to the placebo group, respectively. This is also illustrated in the plots correspond with individual changes from baseline at end of treatment (T84) and at follow-up (T114) (Figure 4).
Overall, LAP reduced with on average 17.8 units and 18.9 units at the end of treatment and at the follow-up visit compared to baseline, respectively, but there was no evidence of a treatment effect (F-value = 2.1, p= 0.148). Finally, for HSI, no evidence of treatment effect (F-value = 0.2, p= 0.677) or a period effect was found (F-value = 2.1, p= 0.085).
In summary, BMI, WC, and HSI did not change in function of period and no differences were observed between the treatments. TG, FPG, ALT and LAP reduced in comparison to baseline, however this reduction was similar for the placebo and combined nutraceutical group. HDL increased in comparison with baseline, however this increase was similar for the placebo and combined nutraceutical group. AST, gamma-GT, and FLI reduced at the end of treatment and at follow-up, this reduction was significantly higher for the group that received the combined nutraceutical.
Figure 2: Individual profile plots (black) and mean trend (blue) for AST
Figure 3: Individual profile plots (black) and mean trend (blue) for gamma-GT.
Figure 4: Individual profile plots (black) and mean trend (blue) for FLI
Discussion
There is a growing interest in the study of nutraceuticals with hepatoprotective activities as confirmed in several randomized controlled clinical trials [38]. This trend could be explained to the large increase in the incidence of NAFLD; in fact, it is well known that this condition is the most common cause of chronic liver disease in Western countries [38]. Its clinical burden is not only confined to liver-related morbidity and mortality, but there is now growing evidence that NAFLD is a multisystem disease, affecting extra-hepatic organs and regulatory pathways. For example, NAFLD increases risk of type 2 diabetes, cardiovascular diseases, and chronic kidney disease [39]. Even if the main preventive/therapeutic tool of NAFLD is currently the improvement of lifestyle, most of the time is not enough and could be adjuvanted with nutraceuticals [40].
In our study, the tested association, induced a significant improvement in NAFLD biomarkers (FLI, gamma-GT, AST) compared to placebo, in association with a standardized stabilization diet. Liver detoxification is an essential process in our lives as we are constantly exposed to toxic substances. These substances originate from air pollution, water pollution, alcohol, medicine intake, pesticides etc. [41]. Detoxification is a complicated process composed of three phases to make the apolar toxic molecule water soluble and ready for excretion. These three detoxification phases are functionalization, conjugation and elimination. During the functionalization phase, a functional group is placed on the apolar toxin via cytochrome P450 enzymes and free radicals are captured. In the next phase, this intermediate product is quickly converted - by conjugation with a molecule - to make it water soluble as this intermediate is often more reactive than the original molecule [42]. This polar end-product is afterwards excreted via urine or via bile in feces. The above liver detoxification process might be positively influenceable by providing the right vitamins, minerals and co-factors to the body via e.g. the intake of the tested product.
Choline is important to phospholipid after phosphorylation and donor of methyl-groups after oxidation, and it’s shown that choline-deprived humans suffer from hepatosteatose and liver cell death as choline is an essential nutrient metabolized in the liver [43, 44]. Choline (via phospholipids synthesis) next to Vitamins B2, B3, B6 and B12, folic acid and omega-3 (alpha-lipoic acid) play an important role in phase I liver detoxification as well as Vitamins A, C and E, Selenium, copper, zinc, Manganese, flavonoids (from plant extracts) and Molybdenum [45, 46].
N-Acetyl-L-Cysteine (L-NAC) acts outside the cell to reduce cystine to cysteine which is 10 times faster transported in the cell and which can be used for the biosynthesis of glutathione (GSH) [47]. In the biosynthesis of GSH, NAC plays an indirect anti-oxidative role by enhancing the Glutathion-S-transferase activity and by delivering GSH for the glutathionperoxidase catalyzed detoxification of peroxides. NAC can also capture free radicals and reacts strongly with HOCL. It is also able of reducing HO and H2O2 [48]. This anti-oxidative quality is very useful after Phase I of liver detoxification.
In phase II, characterized of the conjugation of the intermediate with a molecule (glucuronidation, sulfation, methylation, amino acid conjugation, glutathione conjugation, and acetylation), methylation is supported by nutrient cofactors and methyl donors such as folate and vitamin B12 [49]. Other components supporting conjugation are glutathione and NAC for glutathion conjugation, sulfur for sulfation and co-factors glycine, glutamine and taurine [50].
For both Phase I and Phase II, selective induction or modification of metabolic enzymes is supported by watercress, catechins from green tea and Ellagic acid from pomegranate. They inhibit the overproduction of phase I enzymes and stimulate the production of phase II enzymes in order to quickly convert the active metabolite formed after phase I [51-53]. Next to this, catechins and polyphenols from green tea, Cynarine from artichoke leaves and phytochemicals from pomegranate have also an anti-oxidative effect [54, 55]. Silymarin from the milk thistle has a double effect on this pathway. On one hand, it increases serum glutathion and glutathione peroxidase stimulating phase II and on the other hand, silymarin glucosides have strong antioxidant characteristics [56]. Furthermore, Artichoke is known as hepatoprotectant, antioxidant and reducer of glutathione loss [57].
Phase III – excretion - is supported on the urine and on the bile level. On the urine level, Caffeine from green tea acts as a diuretic (20) and urinary PH is elevated with potassium citrate [58]. On the bile level, bile production is stimulated with cholagogue and choleretic plant extracts from artichoke [29].
Next to the support of liver detoxification and the anti-oxidative characteristics of many substances, others support mainly general liver function. Lysine and threonine are essential amino acids needed for the metabolism of fatty acids in the liver as deficiencies accompany the development of a fatty liver [60]. Next to this, Lysine acetylation is used for regulation of different processes in the body e.g. the regulation of the glycolysis, gluconeogenesis, Citric acid cycle, urea cycle, glycogen and fatty acid metabolism influencing the health status of the liver [61]. The glucose and lipid metabolism are also influenced by biotin. Biotin reduces hypertriglyceridemia, triacylglycerol and VLDL, especially when lipid levels are elevated, which reduces risk of development of fatty liver disease and attenuated hepatotoxicity [62]. Vitamin D plays a role as regulator of glucose and lipid metabolism, inflammation, cellular proliferation, differentiation and apoptosis in the liver, as well. Studies have shown the link between vitamin D deficiency and chronic liver disease as well as the improvement in hepatic inflammation and fibrosis via vitamin D in Non-alcoholic Fatty liver disease [63]. This is also modified by pantethine, a derivate of vitamin B5 and the active site of CoA. Pantethine pools triglycerides in the body as hepato-visceral and subcutaneous fat instead of storing it in the liver while CoA is used for acetylating and amino acid conjugation in phase II of liver detoxification [64]. In addition, Vitamin B1 or Thiamine deficiencies are commonly found in patients with chronic liver disease [65]. Thiamine is converted in the body to Thiamine pyrophosphate, a co-factor in intermediary metabolism but can also be used as essential co-enzyme for glucose utilization, both beneficial for the liver [66].
As deficiencies of most of above-mentioned nutrients are commonly seen in patients with NAFLD, it is important to provide them with a full spectrum supplement not only supporting the detoxification of the liver but also the general liver function in order to overall sustain the liver function.
This study has some limitations that have to be considered in the evaluation of the proposed results. Firstly, the sample size was relatively small, even though sufficiently powered for the aim of the study. Secondly, the duration of exposure was relatively short, but still useful to evaluate the positive effects of the tested nutraceutical on NAFLD biomarkers and its excellent tolerability in the short term. In addition, due to the high complexity of the tested formulation for to the large number of molecules present in it, it is difficult to separate and understand the efficacy of each single molecule as well as the related pharmacokinetic and pharmacodynamic profiles that might be altered due to interference with other substances contained in the tested product.
In conclusion, the tested nutraceutical association has proven to effectively and safely improve liver steatosis markers in the short-middle term. This result should be confirmed in long-term larger studies.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 02, Feb 2019Accepted: Fri 08, Mar 2019
Published: Mon 18, Mar 2019
Copyright
© 2023 Arrigo F.G. Cicero. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2018.01.004
Author Info
Federica Fogacci Alessandro Colletti An-Katrien Vynckier Arrigo F.G. Cicero Claudio Borghi Laura Garcia-Molina Maddalena Veronesi Maurizio Salamone Mieke Van Den Driessche
Corresponding Author
Arrigo F.G. CiceroDepartment of Medicine and Surgery Sciences, University of Bologna, Bologna, Italy
Figures & Tables
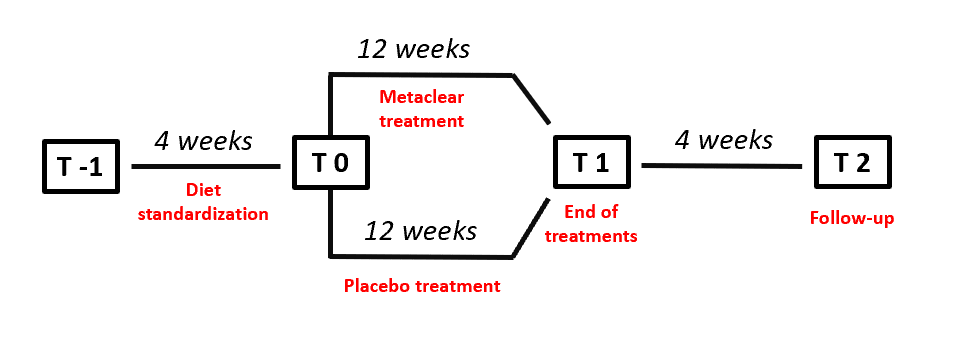
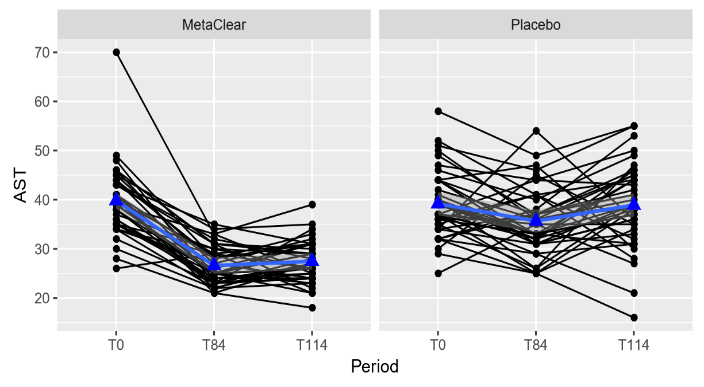
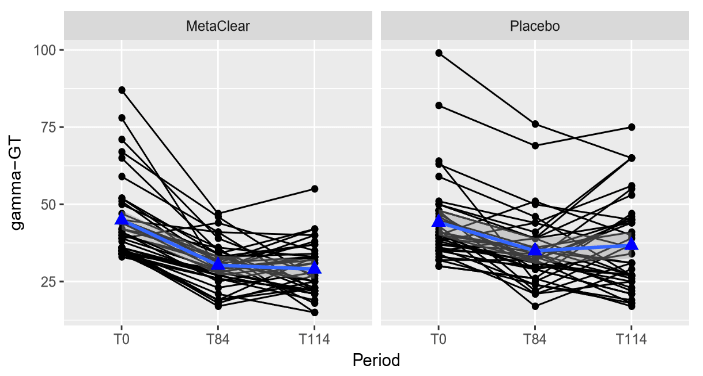
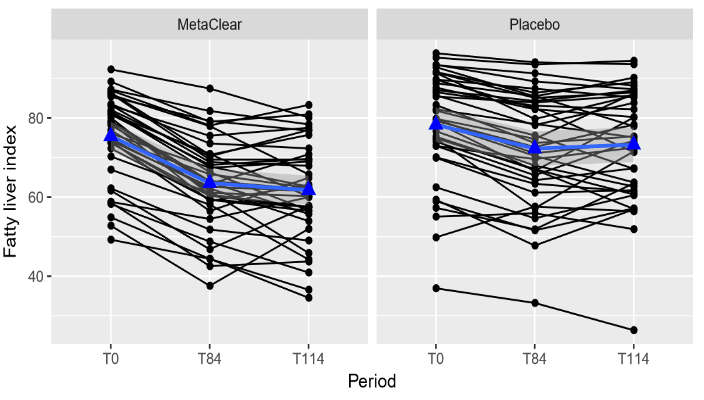
Table 1: Description of the tested product
|
%RI* |
|||
|
Vitamin A (retinyl palmitate) |
125 µg |
16% |
|
|
Vitamin B1 (thiamine monohydrate) |
2,1 mg |
191% |
|
|
Vitamin B2 (riboflavin) |
2,4 mg |
171% |
|
|
Vitamin B3 (niacinamide, niacin) |
11,7 mg |
73% |
|
|
Vitamine B5 (calcium pantothenate) |
9,0 mg |
150% |
|
|
Vitamine B6 (pyridoxal-5-phosphate) |
3,0 mg |
214% |
|
|
Vitamine B12 (methylcobalamine) |
25 µg |
100% |
|
|
Vitamine C (magnesium ascorbate) |
66,67 mg |
83% |
|
|
Vitamine D (cholecalcipherol) |
25 µg |
500% |
|
|
Vitamine E (D-alpha tocopheryl acetate) |
17,01 mg |
142% |
|
|
Biotin |
33,30 µg |
67% |
|
|
Folate (calcium-L-methylfolate) |
134 µg |
67% |
|
|
Copper (copper citrate) |
333 µg |
33% |
|
|
Manganese (manganese citrate) |
0,83 mg |
42% |
|
|
Molybdenum (sodium molybdate) |
33,3 µg |
67% |
|
|
Selenium (selenium methionine) |
25 µg |
45% |
|
|
Zinc (zinc citrate) |
3.3 mg |
33% |
|
|
Artichoke plant extract (Cynara scolymus) |
75 mg |
||
|
Contains cynarine |
3,75 mg |
||
|
Green tea leaf extract (Camellia sinensis) |
50 mg |
||
|
Contains: polyphenols |
Min 25 mg |
||
|
Catechins |
10-15 mg |
||
|
epigallocatechin-3-gallate |
3,5-6,5 mg |
||
|
Caffein |
2-3 mg |
||
|
Milk thistle seed extract (Silybum marianum) |
100 mg |
||
|
Contains silymarine |
80 mg |
||
|
Pomegranateextract (Punica granatum) |
100 mg |
||
|
Contains ellagic acid |
40 mg |
||
|
Watercress leaf extract (Nasturtium officinale) |
100 mg |
||
|
N-acetyl-L-cysteine |
150 mg |
||
|
Choline |
82,5 mg |
||
|
Alpha-lipoic acid |
50 mg |
||
|
L-cysteine HCl |
5 mg |
||
|
L-glutathione |
10 mg |
||
|
L-glycine |
400 mg |
||
|
L-lysine HCl |
35 mg |
||
|
L-threonine |
35 mg |
||
|
Potassium citrate |
214 mg |
||
|
Taurin |
100 mg |
||
|
Sodium sulfate |
50 mg |
||
|
*RI = Reference Intake |
|||
Table 2: Patient characteristics at baseline (data reported as mean and standard deviation)
|
|
Placebo |
Combined nutraceutical |
|
Placebo |
Combined nutraceutical |
|
Age (years) |
57.4 (13.6) |
59.6 (12.5) |
Total Cholesterol (mg/dL) |
224.4 (38.5) |
221.3 (34.8) |
|
Systolic blood pressure (mmHg) |
143.3 (20.4) |
141.5 (17.9) |
Triglycerides (mg/dL) |
189.1 (98) |
199.3 (152) |
|
Diastolic blood pressure (mmHg) |
78 (9.4) |
75 (7.7) |
HDL-Cholesterol(mg/dL) |
42.5 (11.5) |
39.4 (11.9) |
|
Waist circumference (cm) |
103.4 (7.6) |
100.5 (5.9) |
LDL-Cholesterol (mg/dL) |
143.8 (33.9) |
148.2 (34.9) |
|
Body Mass Index (kg/m2) |
29.8 (2.9) |
28.9 (2.4) |
Fasting Plasma Glucose (mg/dL) |
97.7 (11.6) |
95.3 (11.8) |
Table 3: Liver parameters at the baseline in both studied groups (data reported as mean and standard deviation)
|
|
Placebo |
Combined nutraceutical |
|
Placebo |
Combined nutraceutical |
|
Aspartate transaminase (AST) |
39.2 (6.9) |
39.8 (7.2) |
Fatty Liver Index (FLI) |
87.5 (11.1) |
88 (8.9) |
|
Alanine transaminase (ALT) |
41.4 (9.7)
|
41.9 (10.3)
|
Hepatic Steatosis Index (HIS) |
31.6 (2.8) |
30.6 (3.1) |
|
gamma-Glutamyl Transferase |
44.2(13.6) |
44.9 (12.9)
|
Lipid Accumulation Product (LAP) |
84.1 (43.2) |
78.5 (50.1) |
References
- Chatalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological association. Hepatology 55: 2005-2023.
- Le MH, Devaki P, Ha NB, Jun DW, Te HS, et al. (2017) Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One 12: e0173499. [Crossref]
- Fan JG, Zhu J, Li XJ, Chen L, Li L, et al. (2005) Prevalence of and risk factors for fatty liver in a general population of Shangai, China. J Hepatol 43: 508-514. [Crossref]
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, et al. (2006) The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6: 33. [Crossref]
- Villela Nogueira CA, Leite NC, Cardoso CR, Salles GF (2016) NAFLD and increased aortic stiffness: parallel or common physiopathological mechanisms? Int J Mol Sci 17: 460. [Crossref]
- Calzadilla Bertot L, Adams LA (2016) The natural course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci 17: 774. [Crossref]
- Benedict M, Zhang X (2017) Nonalcoholic fatty liver disease. An expanded review. World J Hepatol 9: 715-732. [Crossref]
- Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM (2017) Nonalcoholic Fatty Liver Disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr 11: S209-S216. [Crossref]
- Asgari Taee F, Zerafati Shoae N, Dehghani M, Sadeghi M, Baradaran HR (2018) Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur J Nutr 14. [Crossref]
- Wijarnpreecha K, Thongprayoon C, Edmonds PJ, Cheungpasitporn W (2016) Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: A systematic review and meta-analysis. QJM 109: 461-466. [Crossref]
- Suárez M, Boqué N, Del Bas JM, Mayneris Perxachs J, Arola L (2017) Mediterranean diet and multi-ingredient-based interventions for the management of nonalcoholic fatty liver disease. Nutrients 9: 1052. [Crossref]
- Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P (2017) Insomnia and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J Postgrad Med 63: 226-231. [Crossref]
- Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G (2016) Fatty liver disease and lifestyle in youngsters: Diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int 36: 427-433. [Crossref]
- He W, An X, Li L, Shao X, Li Q, et al. (2017) Relationship between hypothyroidism and Non-Alcoholic Fatty Liver Disease: A systematic review and meta-analysis. Front Endocrinol 8: 335. [Crossref]
- Vizuete J, Camero A, Malakouti M, Garapati K, Gutierrez J, et al. (2017) Perspectives on nonalcoholic fatty liver disease: an overview of present and future therapies. J Clin Transl Hepatol 5: 67-75. [Crossref]
- Cicero AF, Colletti A (2016) Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 23: 1134-1144. [Crossref]
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 252: 207-274. [Crossref]
- Ma J, Hennein R, Liu C, Long MT, Hoffmann U, et al. (2018) Improved diet quality associates with reduction in liver fat-particularly in individuals with high genetic risk scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 155: 107-117. [Crossref]
- Trovato FM, Catalano D, Martines GF, Pace P, Trovato GM (2015). Mediterraneandietandnon-alcoholicfatty liver disease: The need of extended and comprehensive interventions. Clin Nutr 34: 86-88. [Crossref]
- Shen H, Rodriguez AC, Shiani A, Lipka S, Shahzad G, et al. (2016) Association between caffeine consumption and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Therap Adv Gastroenterol 9: 113-120. [Crossref]
- Qiu S, Cai X, Sun Z, Li L, Zügel M, et al. (2017) Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Therap Adv Gastroenterol 10: 701-713. [Crossref]
- Orci LA, Gariani K, Oldani G, Delaune V, Morel P, et al. (2016) Exercise-based Interventions for Nonalcoholic Fatty Liver Disease: A Meta-analysis and Meta-regression. Clin Gastroenterol Hepatol 14: 1398-1411. [Crossref]
- Cicero AFG, Colletti A, Bellentani S (2018) Nutraceutical Approach to Non-Alcoholic Fatty Liver Disease (NAFLD): The Available Clinical Evidence. Nutrients 10: E1153. [Crossref]
- Hackett ES, Twedt DC, Gustafson DL (2013) Milk thistle and its derivative compounds: a review of opportunities for treatment of liver disease. J Vet Intern Med 27: 10-16. [Crossref]
- Liu CS, Zheng YR, Zhang YF, Long XY (2016) Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 109: 274-282. [Crossref]
- López Lluch G, Del Pozo Cruz J, Sánchez Cuesta A, Cortés Rodríguez AB, Navas P (2019) Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 57: 133-140. [Crossref]
- Sanidad KZ, Sukamtoh E, Xiao H, McClements DJ, Zhang G (2019) Curcumin: Recent Advances in the Development of Strategies to Improve Oral Bioavailability. Annu Rev Food Sci Technol 11. [Crossref]
- Takaki A, Kawai D, Yamamoto K (2013) Multiple hits, Including oxidative stress as pathogenesis and treatment target in nonalcoholic steatohepatitis (NASH). Int J Mol Sci 14: 20704-20728. [Crossref]
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, et al. (2006) The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6: 33. [Crossref]
- Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469-480. [Crossref]
- Cicero AFG, Colletti A, Fogacci F, Bove M, Giovannini M, et al. (2018) Is it Possible to Significantly Modify Blood Pressure with a Combined Nutraceutical on Top of a Healthy Diet? The Results of a Pilot Clinical Trial. High Blood Press Cardiovasc Prev 25: 401-405. [Crossref]
- Cicero AFG, Fogacci F, Bove M, Giovannini M, Veronesi M, et al. (2019) Short-Term Effects of Dry Extracts of Artichokeand Berberis in Hypercholesterolemic Patients Without Cardiovascular Disease. Am J Cardiol 123: 588-591. [Crossref]
- Bedogni G, Kahn HS, Bellentani S, Tiribelli C (2010) A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol 10: 98. [Crossref]
- Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, et al. (2010) Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 42: 503-508. [Crossref]
- Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, et al. (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6: 33. [Crossref]
- Pinheiro J, Bates D, Deb Roy S, Sarkar DR (2017) Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3: 1-131.
- R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Mahfood Haddad T, Hamdeh S, Kanmanthareddy A, Alla VM (2017) Nonalcoholic Fatty Liver Disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab Syndr 11: S209-S216. [Crossref]
- Benedict M, Zhang X (2017) Nonalcoholic fatty liver disease. An expanded review. World J Hepatol 9: 715-732. [Crossref]
- Suárez M, Boqué N, Del Bas JM, Mayneris Perxachs J, Arola L, et al. (2017) Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 9: E1052. [Crossref]
- Allen J, Montalto M, Lovejoy J, Weber W (2011) Detoxification in naturopathic medicine: a survey. J Altern Complement Med 17: 1175-1180. [Crossref]
- Hodges RE, Minich DM (2015) Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab 2015: 760689. [Crossref]
- Zeisel SH (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26: 229-250. [Crossref]
- Corbin KD, Zeisel SH (2012) Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 28: 159-165. [Crossref]
- Ale Ebrahim M, Eidi A, Mortazavi P, Tavangar SM, Tehrani DM (2015) Hepatoprotective and antifibrotic effects of sodium molybdate in a rat model of bile duct ligation. J Trace Elem Med Biol 29: 242-248. [Crossref]
- Main PA, Angley MT, O'Doherty CE, Thomas P, Fenech M (2012) The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: a systematic review and meta-analysis. Nutr Metab (Lond) 9: 35. [Crossref]
- Whillier S, Raftos JE, Chapman B, Kuchel PW (2009) Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep 14: 115-124. [Crossref]
- Moldéus P, Cotgreave IA (1994) N-acetylcysteine. Methods Enzymol 234: 482-492. [Crossref]
- Hodges RE, Minich DM (2015) Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab 2015: 760689. [Crossref]
- Hodges RE, Minich DM (2015) Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab 2015: 760689. [Crossref]
- Faria A, Monteiro R, Azevedo I, Calhau C (2007) Pomegranate juice effects on cytochrome P450S expression: in vivo studies. J Med Food 10: 643-649. [Crossref]
- Brown MD (1999) Green tea (Camellia sinensis) extract and its possible role in the prevention of cancer. Altern Med Rev 4: 360-370. [Crossref]
- Chen L, Mohr SN, Yang CS (1996) Decrease of plasma and urinary oxidative metabolites of acetaminophen after consumption of watercress by human volunteers. Clin Pharmacol Ther 60: 651-660. [Crossref]
- Gebhardt R (1997) Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara scolymus L.) against hydroperoxide-induced oxidative stress in cultured rat hepatocytes. Toxicol Appl Pharmacol 144: 279-286. [Crossref]
- Heber D, Seeram NP, Wyatt H, Henning SM, Zhang Y (2007) Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J Agric Food Chem 55: 10050-10054. [Crossref]
- Féher J, Lengyel G (2012) Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol 13: 210-217. [Crossref]
- Kraft K (1997) Artichoke leaf extract - Recent findings reflecting effects on lipid metabolism, liver and gastrointestinal tracts. Phytomedicine 4: 369-378. [Crossref]
- Pak CY, Sakhaee K, Fuller C (1996) Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int 30: 422-428. [Crossref]
- Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek Gärtner H, Petrowicz O, et al. (1994) Increase in choleresis by means of artichoke extract. Phytomedicine 1: 107-115. [Crossref]
- Viviani R, Sechi AM, Lenaz G (1966) Lipid metabolism in fatty liver of lysine- and threonine-deficient rats. J Lipid Res 7: 473-478. [Crossref]
- Kim J, Lee KS, Kwon DH, Bong JJ, Jeong JY, et al. (2014) Severe dietary lysine restriction affects growth and body composition and hepatic gene expression for nitrogen metabolism in growing rats. J Anim Physiol Anim Nutr (Berl) 98: 149-157. [Crossref]
- Marshall MW, Kliman PG, Washington VA, Mackin JF, Weinland BT (1980) Effects of biotin on lipids and other constituents of plasma of healthy men and women. Artery 7: 330-351. [Crossref]
- Eliades M, Spyrou E (2015) Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol 21: 1718-1727. [Crossref]
- Shibata K, Fukuwatari T, Higashiyama S, Sugita C, Azumano I, et al. (2013) Pantothenic acid refeeding diminishes the liver, perinephrical fats, and plasma fats accumulated by pantothenic acid deficiency and/or ethanol consumption. Nutrition 29: 796-801. [Crossref]
- Rossouw JE, Labadarios D, Krasner N, Davis M, Williams R (1978) Red blood cell transketolase activity and the effect of thiamine supplementation in patients with chronic liver disease. Scand J Gastroenterol 13: 133-138. [Crossref]
- Hassan R, Qureshi H, Zuberi SJ (1991) Effect of thiamine on glucose utilization in hepatic cirrhosis.J Gastroenterol Hepatol 6: 59-60. [Crossref]
