Discordance of HER2 Positive Classic Invasive Lobular Carcinoma Between IHC/FISH and Oncotype DX: A Case Report and Literature Review
A B S T R A C T
The classical-type of invasive lobular carcinoma (ILC) usually expresses high levels of estrogen receptor (ER) and progesterone receptor (PR) and does not over-express human epidermal growth factor receptor 2 (HER2) and has a low level of expression of the proliferative marker. Their prognosis is comparable with those of histologic grade 1-2 invasive ductal carcinoma (IDC). HER2 status is prognostically significant and has a huge impact on clinical management; thus, its correct testing and evaluation are critical. Discordant results for HER2 between immunohistochemistry (IHC)/fluorescence in situ hybridization (FISH) and Oncotype DX (ODX) testing can be occasionally seen and cause confusion among clinicians. Here, we present a case of classical ILC (cILC), which was HER2 positive by IHC/FISH but negative by ODX. We also review relevant literature and provide a possible explanation for this discordancy and suggestions on how to approach the discordant results and guide the clinical management.
Keywords
Invasive lobular carcinoma, HER2, oncotype DX
Introduction
Invasive lobular carcinoma (ILC) is the most common invasive breast carcinoma of special types, consists of 10-15% of breast cancer and its incidence rate is rising [1-3]. Despite the often favourable clinical and pathological features such as older patients, lower grade, ER and HER2 negative, and lower Ki67, these tumors have a higher risk of distance recurrence [4, 5]. Majority of the classical-type invasive lobular carcinoma (cILC) express estrogen receptor (ER) and progesterone receptor (PR), but no overexpression or amplification of human epidermal growth factor receptor 2 (HER2) [6, 7]. Although rare, HER2 positivity can be seen in cILC, around 5-6%, and is associated with a worse prognosis, more so than that of invasive ductal carcinoma (IDC) [8-11]. More importantly, HER2 positivity makes these subgroups of cILC eligible for HER2 targeted therapy, and it benefits similarly to IDC [12, 13].
Two clinically validated HER2 testings available are immunohistochemistry (IHC) analysis and fluorescence in situ hybridization (FISH) testing [14-16]. In 2018, College of American Pathologists (CAP)/American Society of Clinical Oncology (ASCO) updated the HER2 testing guideline and eliminated the HER2 equivocal group [17]. Oncotype DX (ODX) is a test that uses reverse transcription-polymerase chain reaction (RT-PCR) to predict the distant recurrence rate and assigns a proprietary recurrence score (RS). It has been validated for use on ER/PR positive, HER2 negative, and node-negative breast cancer, and to assess the benefit of adjuvant chemotherapy [18]. Since 2008, besides reporting RS, ODX also started to report the status of the tumor markers, namely ER, PR, and HER2, although the use of these tumor markers has not been clinically validated [19]. Here, we report a case of cILC with discordant HER2 status between HER2 IHC/FISH and ODX, which caused confusion for the clinicians on how to treat the patient.
Case Presentation
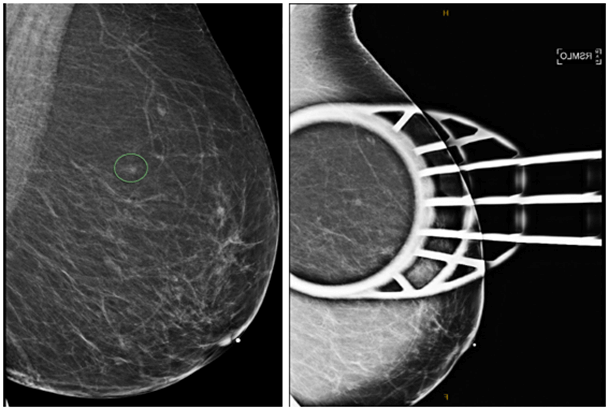
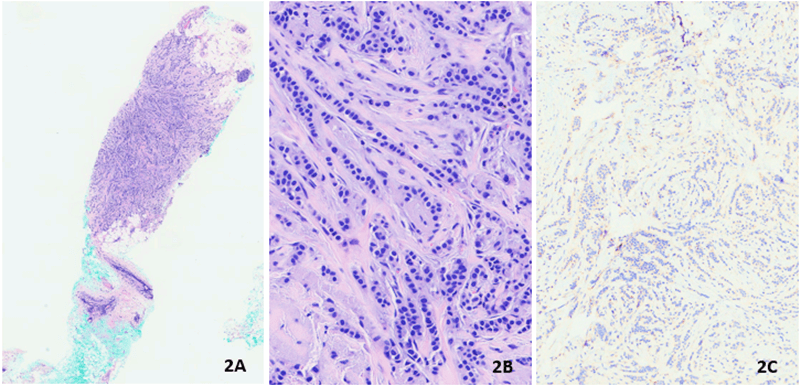
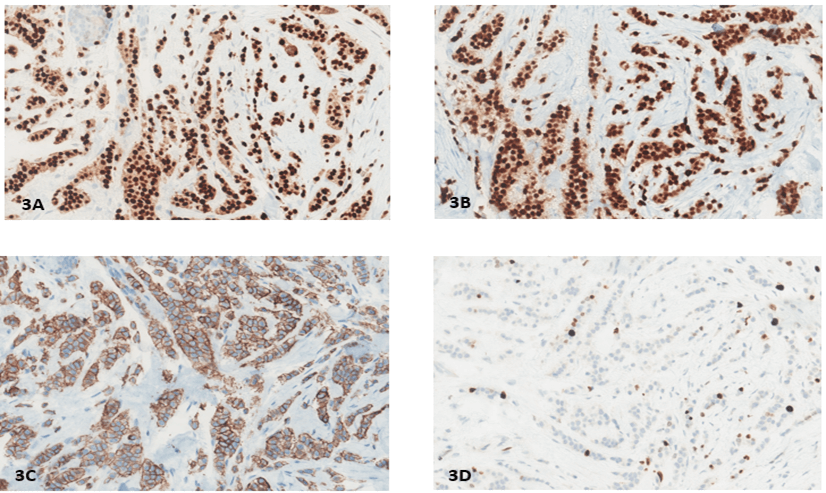
Our patient was a 70-year-old woman with no significant past medical history who underwent a screening mammogram in June 2020 that showed an indeterminate asymmetry in her right breast. The subsequent diagnostic mammogram showed previously described right superior focal asymmetry, partially effaced upon spot compression view, and was reproducible on both full-field spot compression imaging with a suggestion of a subtle mammographic pattern of architectural distortion (Figure 1). Ultrasound-guided biopsy was performed and showed invasive lobular carcinoma, a classical-type that was confirmed with loss of E-Cadherin IHC stain (Figures 2A-2C). IHC stains for ER, PR, HER2, and KI67 were performed and showed strong expression for ER and PR (Allred Score 8), over-expression for HER2 (3+), and low proliferative index for Ki67 (6%). (Figures 3A-3D). HER2 FISH analysis was also ordered and confirmed HER2 amplification with a ratio of 3.2 with the average number of HER2 copy number 9.8. The patient then underwent a lumpectomy, which revealed biopsy confirmed invasive lobular carcinoma, classical-type, 0.7 cm in size, with negative margins and one negative sentinel lymph node (LN). A second pathology opinion of her biopsy and lumpectomy specimens at UCS Norris confirmed our IHC and FISH findings. Per patient request, ODX testing was obtained which showed RS 18, with 5% distant recurrence in 9 years and <1% chemotherapy benefit. The test also reported this tumor as ER-positive (9.5), PR positive (7.0) but HER2 negative (10.1), which differed from both IHC and FISH results. After extensive discussion at tumor board, and with the patient, she elected to undergo adjuvant paclitaxel and trastuzumab therapy as per the APT protocol [20]. She started adjuvant anastrozole and currently, there is no evidence of recurrent disease.
Figure 1: Subtle mammographic pattern of architectural distortion on spot compression mammogram.
Figure 2: Biopsy showing invasive lobular carcinoma A) H&E, B) 4x, C) 40x, E. Cadherin stain.
Figure 3: A) ER Strong positive, Allred 8; B) PR Strong positive, Allred 8; C) Her2 positive (3+); D) Ki-67 6%.
ER: Estrogen Receptor; PR: Progesterone Receptor; HER2: Human Epidermal Growth Factor Receptor 2.
Discussion
i. Several studies have shown that cILC can be HER2 positive at a rate between 5-6%, which are comparable with our own unpublished data [8-11]. HER2 positivity is associated with older patients, PR negativity, lower ER expression, higher Ki67 expression, and more angiolymphatic invasion. Since HER2 positivity is uncommon for cILC, we recommend confirming it with both test modalities (IHC and FISH).
ii. Although the status of ER, PR, and HER2 have been reported by ODX since 2008, which has generated unnecessary confusion among clinicians, especially for HER2 positive cases; as its discordant rate is rather high [19]. The original study for HER2 by Baehner et al., published on the ODX testing website, showed the overall concordance is 95% between central IHC and ODX testing among their 729 cases (Table 1) [21]. The concordance for IHC negative cases is high, 99%; however, concordance for IHC positive cases is only 78%. In other words, over 20% of the HER2 IHC positive cases are negative by ODX. Subsequent studies have shown the presence of discordance HER2 status between IHC/FISH and ODX testing, especially in HER2 IHC/FISH positive cases, the rates are between 0 and 50% [22-27]. These studies were summarized in (Table 2). These studies demonstrated that high overall concordance (>95%) between IHC/FISH and ODX for HER2 testing is largely from the overwhelming cases of HER2 negative tumors; while the HER2 positive tumor demonstrated unacceptably high discordance (0-50%). ODX was originally designed only for ER-positive, HER2-negative, and LN-negative breast tumors for recurrence score. Tumor markers status from ODX is not clinically validated and should not be used as a guide for clinical decision making.
Table 1: Concordance rate between IHC stain and ODX modified
and adopted from Baehner et al. [21]
|
|
IHC+ |
IHC- |
Total |
|
RT-PCR by ODX + |
94 (78%) |
4 (1%) |
98 |
|
RT-PCR by ODX + |
27 (22%) |
439 (99%) |
631 |
|
Total |
121 |
443 |
729 |
iii. The four groups of biomarkers that ODX used to access the RS of ER-positive, HER2-negative, and LN-negative breast cancers are five proliferation-related genes including Ki67, four estrogen-related genes including ER, and two HER2-related genes including HER2, and two invasion-related genes. The expression of proliferation marker Ki67 is usually very low in ILC, especially for cILC. Cargognin et al. reported that 4%, instead of 20%, which is used for IDC, is a meaningful cutoff for ILC to be prognostically significant [28]. This raises the question of if a similar cut-off for RS should be used for both ductal and lobular carcinoma. Indeed, several studies have shown that ODX is not suitable for the evaluation of ILC, and adjuvant chemotherapy does not confer a survival benefit for intermediate or high-risk patients [29, 30]. A large prospective study on this special but increasingly important subtype of breast cancer shall give us further answers to this.
Table 2: Summary of concordant rates from literature review.
|
Total cases |
Total IHC/FISH positive cases |
HER 2 IHC/FISH positive concordance |
Total IHC/FISH negative/equivocal cases |
HER2 IHC/FISH negative concordance |
Overall HER2 concordance |
|
|
Sughayer et al. 2020 [22] |
113 |
0 |
ND |
113 |
99.1% |
99.1% |
|
Chang et al. 2018 [24] (separate IHC and FISH results) |
30 |
0 |
ND |
30 |
100 (IHC)/93.3 (FISH)% |
100/93.3% |
|
Neely et al. 2018 [23] |
610 |
5 |
20% |
558 |
100% |
98.6% |
|
Park et al. 2014 [25] (FISH only) |
265 |
2 |
0% |
245 |
100% |
99.2% |
|
Dvorak et al. 2013 [26] (FISH only) |
194 |
8 |
50% |
186 |
100% |
96% |
|
Dabbs et al. 2011 [27] |
843 |
36 |
42% |
784 |
99% |
98% |
IHC: Immunohistochemistry; ODX: Oncotype DX; RT-PCR:
Reverse Transcription-Polymerase Chain Reaction; IHC+: Positive by IHC stain;
IHC-: Negative by IHC stain; FISH: Fluorescence In Situ Hybridization; HER2:
Human Epidermal Growth Factor Receptor 2; ND: No Data.
Conclusion
Our case serves as a good example for cILC at several levels: i) although rare, HER2 can be positive in cILC, ii) discordance for HER2 positive breast cancer are very high between IHC/FISH and ODX testing, and iii) clinical management should not be based on tumor markers status from ODX.
Data Availability
All data analyzed during this study are available from the corresponding author on request.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Wed 18, May 2022Accepted: Thu 02, Jun 2022
Published: Tue 14, Jun 2022
Copyright
© 2023 Khin Su Mon. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2022.06.01
Author Info
Khin Su Mon Anthony Wheeler Shelly S. Lo Ping Tang
Corresponding Author
Khin Su MonDepartment of Pathology and Laboratory Medicine, Loyola University Medical Center, Maywood, Illinois, USA
Figures & Tables
Table 1: Concordance rate between IHC stain and ODX modified
and adopted from Baehner et al. [21]
|
|
IHC+ |
IHC- |
Total |
|
RT-PCR by ODX + |
94 (78%) |
4 (1%) |
98 |
|
RT-PCR by ODX + |
27 (22%) |
439 (99%) |
631 |
|
Total |
121 |
443 |
729 |
Table 2: Summary of concordant rates from literature review.
|
Total cases |
Total IHC/FISH positive cases |
HER 2 IHC/FISH positive concordance |
Total IHC/FISH negative/equivocal cases |
HER2 IHC/FISH negative concordance |
Overall HER2 concordance |
|
|
Sughayer et al. 2020 [22] |
113 |
0 |
ND |
113 |
99.1% |
99.1% |
|
Chang et al. 2018 [24] (separate IHC and FISH results) |
30 |
0 |
ND |
30 |
100 (IHC)/93.3 (FISH)% |
100/93.3% |
|
Neely et al. 2018 [23] |
610 |
5 |
20% |
558 |
100% |
98.6% |
|
Park et al. 2014 [25] (FISH only) |
265 |
2 |
0% |
245 |
100% |
99.2% |
|
Dvorak et al. 2013 [26] (FISH only) |
194 |
8 |
50% |
186 |
100% |
96% |
|
Dabbs et al. 2011 [27] |
843 |
36 |
42% |
784 |
99% |
98% |
IHC: Immunohistochemistry; ODX: Oncotype DX; RT-PCR:
Reverse Transcription-Polymerase Chain Reaction; IHC+: Positive by IHC stain;
IHC-: Negative by IHC stain; FISH: Fluorescence In Situ Hybridization; HER2:
Human Epidermal Growth Factor Receptor 2; ND: No Data.
ER: Estrogen Receptor; PR: Progesterone Receptor; HER2: Human Epidermal Growth Factor Receptor 2.
References
1. Li CI, Anderson BO,
Daling JR, Moe RE (2003) Trends in incidence rates of invasive lobular and
ductal breast carcinoma. JAMA 289: 1421-1424. [Crossref]
2. Weigelt B, Horlings
HM, Kreike B, Hayes MM, M Hauptmann et al. (2008) Refinement of breast cancer
classification by molecular characterization of histological special types. J
Pathol 216: 141-150. [Crossref]
3. WHO Classification
of Tumours Editorial Board (2019) The 2019 World Health Organization
classification of tumours of the breast. Lyon (France): International Agency
for Research on Cancer. 5th ed.; 2: 114-118.
4. Flores Díaz D, Arce
C, Flores Luna L, Reynoso Noveron N, Lara Medina F et al. (2019) Impact of
invasive lobular carcinoma on long-term outcomes in Mexican breast cancer
patients. Breast Cancer Res Treat 176: 243-249. [Crossref]
5. Mouabbi JA, Hassan
A, Lim B, Hortobagyi GN, Tripathy D et al. (2022) Invasive lobular carcinoma:
an understudied emergent subtype of breast cancer. Breast Cancer Res Treat
2022: 253-264. [Crossref]
6. Arpino G, Bardou
VJ, Clark GM, Elledge RM (2004) Infiltrating lobular carcinoma of the breast:
tumor characteristics and clinical outcome. Breast Cancer Res 6:
R149-R156. [Crossref]
7. McCart Reed AE,
Kalinowski L, Simpson PT, Lakhani SR (2021) Invasive lobular carcinoma of the
breast: the increasing importance of this special subtype. Breast Cancer Res
23: 6. [Crossref]
8. Rosenthal SI,
Depowski PL, Sheehan CE, Ross JS (2002) Comparison of HER-2/neu oncogene
amplification detected by fluorescence in situ hybridization in lobular and
ductal breast cancer. Appl Immunohistochem Mol Morphol 10: 40-46. [Crossref]
9. Yu J, Dabbs DJ,
Shuai Y, Niemeier LA, Bhargava R (2011) Classical-type invasive lobular
carcinoma with HER2 overexpression: clinical, histologic, and hormone receptor
characteristics. Am J Clin Pathol 136: 88-97. [Crossref]
10. Wang T, Ma Y, Wang
L, Liu H, Chen M et al. (2015) Strong adverse effect of epidermal growth factor
receptor 2 overexpression on prognosis of patients with invasive lobular breast
cancer: a comparative study with invasive ductal breast cancer in Chinese
population. Tumour Biol 36: 6113-6124. [Crossref]
11. Zhang H, Moisini I,
Ajabnoor RM, Turner B, D'aguiar M et al. (2020) Frequency, Clinicopathologic
Characteristics, and Follow-up of HER2-Positive Nonpleomorphic Invasive Lobular
Carcinoma of the Breast: A Retrospective Analysis of an 11-Year Study in an
Academic Institution. Am J Clin Pathol 153: 583-592.
12. Metzger Filho O,
Procter M, de Azambuja E, Leyland Jones B, Gelber RD et al. (2013) Magnitude of
trastuzumab benefit in patients with HER2-positive, invasive lobular breast
carcinoma: results from the HERA trial. J Clin Oncol 31: 1954-1960. [Crossref]
13. Da Ros L, Moretti A, Querzoli P, Pedriali M, Lupini L et al. (2018)
HER2-Positive Lobular Versus Ductal Carcinoma of the Breast: Pattern of First
Recurrence and Molecular Insights. Clin Breast Cancer 18: e1133-e1139. [Crossref]
14. Wolff AC, Hammond
ME, Hicks DG, Dowsett M, McShane LM et al. (2014) Recommendations for human
epidermal growth factor receptor 2 testing in breast cancer: American Society
of Clinical Oncology/College of American Pathologists clinical practice
guideline update. Arch Pathol Lab Med 138: 241-256. [Crossref]
15. Middleton LP, Price
KM, Puig P, Heydon LJ, Tarco E et al. (2009) Implementation of American Society
of Clinical Oncology/College of American Pathologists HER2 Guideline
Recommendations in a tertiary care facility increases HER2 immunohistochemistry
and fluorescence in situ hybridization concordance and decreases the number of
inconclusive cases. Arch Pathol Lab Med 133: 775-780. [Crossref]
16. van de Vijver M,
Bilous M, Hanna W, Hofmann M, Kristel P et al. (2007) Chromogenic in situ
hybridisation for the assessment of HER2 status in breast cancer: an
international validation ring study. Breast Cancer Res 9: R68. [Crossref]
17. Wolff AC, Hammond
ME, Allison KH, Harvey BE, Mangu PB et al. (2018) Human Epidermal Growth Factor
Receptor 2 Testing in Breast Cancer: American Society of Clinical
Oncology/College of American Pathologists Clinical Practice Guideline Focused
Update. Arch Pathol Lab Med 142: 1364-1382. [Crossref]
18. Paik S, Shak S,
Tang G, Kim C, Baker J et al. (2004) A multigene assay to predict recurrence of
tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:
2817-2826. [Crossref]
19. Fox KR (2008) Compact
Guide to Breast Cancer Medical Treatment Available. Oncology 22: 778.
20. Tolaney SM, Barry
WT, Dang CT, Yardley DA, Moy B et al. (2015) Adjuvant paclitaxel and
trastuzumab for node-negative, HER2-positive breast cancer. New Engl J Med
372: 134-141. [Crossref]
21. Baehner FL, Gray R,
Childs BH, Maddala T, Rowley S et al. (2008) HER2 concordance between central
laboratory immunohistochemistry and quantitative reverse transcription
polymerase chain reaction in Intergroup Trial E2197. J Clin Oncol 26:
22009.
22. Sughayer MA,
Alhassoon S, Sughayer HM (2020) Comparison of Estrogen receptors, Progesterone
receptors and HER2-neu immunohistochemistry results in breast cancer with those
of Oncotype Dx. Ann Diagn Pathol 47: 151556. [Crossref]
23. Neely C, You S,
Mendoza PM, Aneja R, Sahin AA et al. (2018) Comparing breast biomarker status
between routine immunohistochemistry and FISH studies and Oncotype DX testing,
a study of 610 cases. Breast J 24: 889-893. [Crossref]
24. Chang CH, Lin YH
(2018) Using Oncotype DX as an additional treatment decision tool in early
breast cancer: a retrospective analysis from a single institution in Taiwan. Ther
Radiol Oncol 2: 7.
25. Park MM, Ebel JJ,
Zhao W, Zynger DL (2014) ER and PR immunohistochemistry and HER2 FISH versus
oncotype DX: implications for breast cancer treatment. Breast J 20:
37-45. [Crossref]
26. Dvorak L, Dolan M,
Fink J, Varghese L, Henriksen J et al. (2013) Correlation between HER2
determined by fluorescence in situ hybridization and reverse
transcription-polymerase chain reaction of the oncotype DX test. Appl
Immunohistochem Mol Morphol 21: 196-199. [Crossref]
27. Dabbs DJ, Klein ME,
Mohsin SK, Tubbs RR, Shuai Y et al. (2011) High false-negative rate of HER2
quantitative reverse transcription polymerase chain reaction of the Oncotype DX
test: an independent quality assurance study. J Clin Oncol 29:
4279-4285. [Crossref]
28. Carbognin L, Sperduti I, Fabi A, Dieci MV, Kadrija D et al. (2017) Prognostic
impact of proliferation for resected early stage ‘pure’ invasive lobular breast
cancer: cut-off analysis of Ki67 according to histology and clinical
validation. Breast 35: 21-26. [Crossref]
29. Kizy S, Huang JL, Marmor S, Tuttle TM, Hui JY (2017) Impact of the 21-gene recurrence score on outcome in patients with invasive lobular carcinoma of the breast. Breast Cancer Res Treat 165: 757-763. [Crossref]
30. Bomeisl PE, Thompson CL, Harris LN, Gilmore HL (2015) Comparison of Oncotype DX Recurrence Score by Histologic Types of Breast Carcinoma. Arch Pathol Lab Med 139: 1546-1549. [Crossref]
