Cecal Perforation in a COVID-19 Patient Treated with Tocilizumab: A Case Report
A B S T R A C T
Tocilizumab is a monoclonal antibody against the interleukin-6 receptor. Gastrointestinal perforation has been associated with Tocilizumab treatment. In the absence of specific antiviral drugs, Tocilizumab is currently being used as a treatment option in patients with severe COVID-19 pneumonia. This is being based on the implication of interleukin-6 in the aberrant host immune response that characterizes the disease and initiates patients' lung damage. Herein we present the case of a cecal perforation in a patient treated with Tocilizumab for COVID-19. In such context, we expect that there may be an increase in the incidence of gastrointestinal perforation related to Tocilizumab.
Keywords
COVID-19, tocilizumab, interleukin-6, gastrointestinal perforation
Introduction
Interleukin-6 (IL-6) is a well-known pro-inflammatory cytokine produced by B and T cells, monocytes and fibroblasts, that mediate several biological functions, including T cell activation, the differentiation of activated B cells into Ig-producing plasma cells, the upregulation of acute-phase protein synthesis in hepatic and adipose tissues. IL-6 is also implicated in the pathogenesis of autoimmune diseases such as Rheumatoid Arthritis (RA) and Juvenile Idiopathic Arthritis [1]. Tocilizumab is an IgG1 recombinant humanized anti-interleukin-6 receptor monoclonal antibody, which is used as a second-line treatment for RA, administered intravenously or subcutaneously, at a dose of 8 mg per Kg every 4 weeks for intravenous administration. It specifically binds to the IL-6 receptor, blocking the IL-6 intracellular signaling pathway [1].
Patients with severe COVID-19 pneumonia present with massive, aberrant and ineffective immune response, mediated by a pro-inflammatory cytokine storm led by IL-6, whose high levels are found in peripheral blood. This hyperimmune response triggers inflammation, exudation, increased vascular permeability and alveolar-capillary membrane edema, compromising gas exchange. In the absence of specific antiviral drugs, Tocilizumab (1-2 intravenous doses of 400 mg) is currently being used to treat patients with severe COVID-19 pneumonia [2].
Case Presentation
We present a case of gastrointestinal perforation (GIP) in a COVID-19 patient treated with Tocilizumab. A 74-year-old female, non-smoker, with no medical history of interest and no usual treatment, presented to the Emergency Department with anorexia, constipation and in an acute confusional state. Clinical examination found the patient with mucosal dryness, crackles in the base of the right lung on auscultation and abdominal examination revealed distention, diffuse tenderness without signs of peritoneal irritation. Laboratory tests showed mild leukopenia and neutropenia with no lymphopenia, thrombocytopenia (102.000/μl), spontaneous coagulopathy, increased D-dimer (18906 μg/l), LDH (991 UI/l), C-reactive protein (7.50 mg/dl), ferritin (2520 ng/ml) and IL-6 (161 pg/ml), along with type 1 respiratory failure (PaO2 60mmHg, PaCO2 27mmHg, PaO2/FiO2 285) in the arterial blood gas.
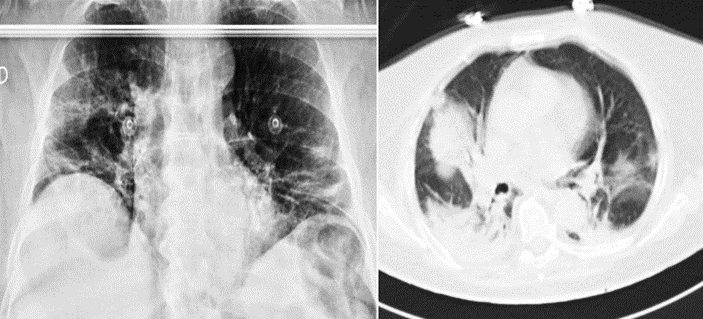
Chest X-ray showed diffuse, hazy pulmonary opacities predominantly involving the right lung suggestive of COVID-19 pneumonia; RT-PCR test and serologic test were therefore performed on the patient and they both resulted positive for SARS-CoV-2. Based on clinical and analytical data, suspecting a multiple organ dysfunction syndrome secondary to intestinal ischaemia computed tomography angiography (CTA) was performed, showing a markedly dilated colon with a fecaloma in the rectum. In addition, CTA showed a normal enhancement of the bowel wall, excluding the suspected diagnosis and furthermore, the scan showed bilateral ground‐glass opacities predominantly involving the right lung, suggestive of COVID-19 pneumonia, as described in the chest X-ray (Figure 1)
Figure 1: Diffuse pulmonary opacities suggestive of COVID-19 pneumonia in chest X-ray and CTA scan.
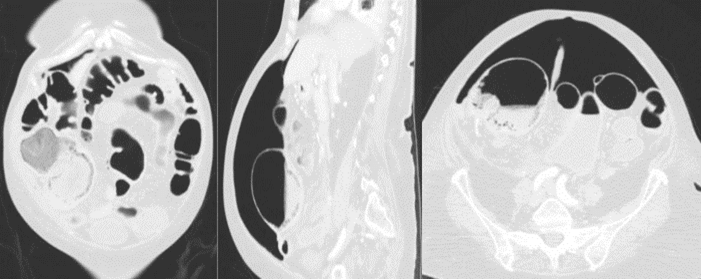
Figure 2: Pneumoperitoneum and intestinal pneumatosis of cecum in CTA scan.
The patient was hospitalized in Internal Medicine to receive treatment for COVID-19 pneumonia, including hydroxychloroquine, high doses of methylprednisolone (initial dose of 250 mg/day per 3 days, then 40 mg/day), lopinavir, ritonavir and Tocilizumab (single IV dose of 400 mg on the first day of hospitalization). Constipation improved with enemas and laxatives. On hospitalization day eight, the patient presented with intense and diffuse abdominal pain with signs of peritoneal irritation. A new CTA was performed, showing a 9 cm dilation of the cecum with intestinal pneumatosis, pericolonic fat stranding, free intraperitoneal fluid and pneumoperitoneum, suggestive of cecal perforation secondary to mesenteric ischaemia (Figure 2). Urgent exploratory laparotomy was performed, finding a cecal perforation of inflammatory aspect with no generalized peritonitis. Right hemicolectomy with terminal ileostomy and the mucous fistula was performed. The postoperative course was uneventful. Microscopically the inflammatory nature of the lesion was confirmed with no signs of malignancy.
Discussion
GIP has been described as a rare but severe complication in relation to Tocilizumab treatment. The incidence rate of GIP in RA patients treated with Tocilizumab, both with subcutaneous and intravenous administration, was 1.2-3.9 per 1000 patient-years, the highest compared with other biological therapies for RA, with an associated mortality of 4.8-46%. No incidence increase was observed with higher drug doses nor longer drug exposure time [3]. Most perforations (71-85%) have occurred in the lower GI tract (small intestine or colon), with an incidence of 2.7 events per 1000 patient-years, HR of 4.48 (95% CI 2.0-10.0), the highest compared to other biological therapies for RA. In such cases, the mortality rate reaches 46%. Previous biological therapies, history of diverticular or peptic ulcer disease, advanced age, tobacco use, glucocorticoid use >7.5mg/day and non-steroidal anti-inflammatory drugs use are well-known predictors of GIP associated with Tocilizumab [3-5]. The physiopathological mechanism of lower GIP in patients with diverticular disease treated with Tocilizumab is explained by the role of IL-6 and its receptor in undertaking intestinal epithelial repair. This is done by stimulating enterocyte proliferation and wound healing and mediating the coverage of an inflamed diverticulum by the surrounding adipose tissue, thus impeding the progression of transmural inflammation and subsequent intestinal perforation, similar to the mesenteric adipose tissue's role in Crohn's disease. Tocilizumab's blockade of the IL-6 pathway can affect this protective mechanism, increasing the risk of GIP [5].
Specifically, we are not able to determine to what degree both Tocilizumab and high doses of glucocorticoids contributed to the pathogenesis of GIP in this case. Although, based on the described physiopathological mechanism, by inhibiting the IL-6 pathway, Tocilizumab may have facilitated the transmural inflammatory progression and the subsequent intestinal perforation, even if its primary cause was the marked cecum dilatation. The half-life t1/2 of Tocilizumab after a single IV dose is 18 days [1]. GIP took place 7 days after its administration. Therefore, its implication in the etiopathology of this case is chronologically plausible.
Conclusion
GIP is a rare but severe complication in relation to Tocilizumab treatment. Although its pathogenic involvement is difficult to prove, given its emerging role as an alternative treatment for patients with severe COVID-19 pneumonia, an increase in the incidence of GIP related to Tocilizumab may occur in such a context.
Funding
This work was supported by Fundación Instituto de Investigación Sanitaria - Fundación Jiménez Díaz (IIS-FJD) (3528/002).
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Tue 27, Jul 2021Accepted: Wed 11, Aug 2021
Published: Thu 09, Sep 2021
Copyright
© 2023 Victor Dominguez Prieto. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.08.21
Author Info
Victor Dominguez Prieto Cecilia Meliga Sara Rosenstone Siyuan Qian Zhang Manuel Escanciano Escanciano Felipe Velez Pinto Sergio Hernandez Villafranca Sara Gonzalez Soares Javier Barambio Buendia Miguel Leon Arellano Damian Garcia Olmo Ricardo Pardo Garcia Hector Guadalajara Labajo
Corresponding Author
Victor Dominguez PrietoSurgery Department, Hospital Universitario Fundacion Jimenez Diaz, Madrid, Spain
Figures & Tables
References
1.
Roche
Registration GmbH (2013) Ficha técnica RoActemra 162 mg solución inyectable en
jeringa precargada. [ficha técnica de medicamento] Madrid: AEMPS.
2.
Fu B, Xu X, Wei H
(2020) Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 18:164. [Crossref]
3.
Monemi S, Berber
E, Sarsour K, Wang J, Lampl K et al. (2016) Incidence of Gastrointestinal
Perforations in Patients with Rheumatoid Arthritis Treated with Tocilizumab
from Clinical Trial, Postmarketing, and Real-World Data Sources. Rheumatol Ther 3: 337-352. [Crossref]
4. Xie F, Yun H, Bernatsky S, Curtis J (2016) Brief Report: Risk of Gastrointestinal Perforation Among Rheumatoid Arthritis Patients Receiving Tofacitinib, Tocilizumab, or Other Biologic Treatments. Arthritis Rheumatol 68: 2612-2617. [Crossref]
5. Strangfeld A, Richter A, Siegmund B, Herzer P, Rockwitz K et al. (2017) Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 76: 504-510. [Crossref]
