Journals
ApoL6: A Novel Biomarker of Apoptotic and Necroptotic Activity in Evolving STsegment Myocardial Infarction in Man
A B S T R A C T
We have previously demonstrated that apolipoprotein L6 (ApoL6) is a pro-death, phospholipid-binding, BH3-only member of the Bcl-2 family. Ectopic expression of ApoL6 induces dichotomous cell death phenotype involving both apoptosis and necroptosis in various cell types. In addition, ApoL6 initiates inflammatory response that upregulates proinflammatory cytokines, such as IL-1β. In this study, we show elevated levels of ApoL6 in the sera of the majority of ST-segment myocardial infarction (STEMI) patients prior to reperfusion which is highly suggestive of the activation of apoptotic and/or necroptotic pathways in ruptured plaque. Thus, ApoL6 could serve as a biomarker and therapeutic target specific for inflammatory apoptotic and/or necroptotic activity in STEMI, as well as other diseases.
Keywords
apolipoprotein L6 (ApoL6), apoptosis, myocardial infarction, necroptosis
Introduction
Coronary arterial atherosclerotic plaque rupture with subsequent intravascular thrombosis is the inciting event prior to the majority of ST-segment myocardial infarctions (STEMI) [1, 2]. Intracellular ApoL6 plays an important role in promoting apoptosis and necroptosis and in inhibiting cytoprotective autophagy in human atherosclerotic lesion cells [3-5]. Plaque rupture is most likely to occur when there is apoptosis and/or necroptosis within the smooth muscle cell component of the thin fibrous cap of advanced lesions [3, 4]. In this proof-of-principle study, we investigated whether ApoL6 can be detected in the circulation, whether ApoL6 expression is elevated in STEMI patients, and whether ApoL6 may serve as a pathway-specific biomarker in STEMI.
Methods
We analysed ApoL6 in plasma samples from 9 patients (P) with evolving STEMI presenting for catheter reperfusion and 5 contemporaneous control subjects (C) presenting for elective coronary angiography. The study has been approved by the Institutional Review Boards as UNM HSC IRB/HRRB protocol #11-059. Written informed consent was obtained from all patients. Blood samples were drawn at baseline when patients were first admitted to the hospital and prior to catheter reperfusion. No STEMI patient had spontaneous reperfusion at admission.
Plasma fractions were separated by size-exclusion/gel filtration chromatography and were used to detect ApoL6, apolipoprotein L1 (ApoL1), and C-reactive protein (CRP) [6]. Lipoprotein and albumin complexes were separated, enriched, and particle-size distribution assessed as previously described [6]. In brief, plasma from a single subject was applied directly to a calibrated Superdex 200 gel filtration column (10/300 GL; GE Healthcare) and the eluate was collected as forty- seven 1.5-mL fractions and maintained at 4 °C. Each fraction was assessed for protein, phospholipid and total cholesterol by colorimetric kits from Wako (Richmond, VA). Western blot analysis was conducted on the fractionated protein samples (#13 to 30) using a home-made anti-ApoL1 and an anti-ApoL6 antibody [3, 7]. Western blot analysis was also conducted on the de-albuminized plasma samples in a standardized way as previously described using an anti-ApoL6 antibody and an anti-CRP antibody (Santa Cruz #sc30047). The duplicate membranes were stained with Ponceau S solution (Cell Signalling #59803) for loading and quality validation.
Results
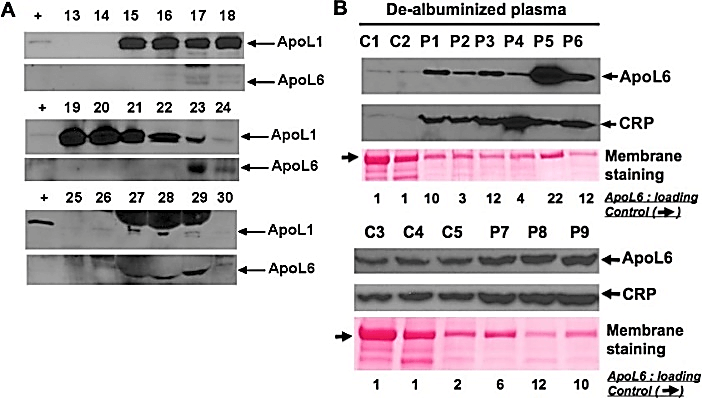
Figure 1A shows that ApoL6 partially co-migrated with ApoL1 (lanes 23 and 24). ApoL6 was also detectable where albumin predominated (lanes 27-29). The densitometric analysis demonstrated that ApoL6 was significantly increased (p = 0.02, Fisher’s exact test) in 7/9 STEMI patients (6 to 22 folds in Figure 1B) as compared to the corresponding controls. Thus, ApoL6 is present to a larger extent than control in STEMI patients prior to reperfusion. Of note, is the presence of CRP in both patients and controls although densitometric analysis shows CRP is present to a larger extent in patient samples (P). The identification of increased ApoL6 in the sera of the majority of STEMI patients prior to reperfusion is highly suggestive of the activation of apoptotic and/or necroptotic.
Figure 1: A) ApoL6 is present in human plasma. Immunoblot analysis of the fractions separated by gel-filtration fractionation showed that ApoL6 was associated with LDL-predominant (lanes 17 & 18), ApoL1-colocalized HDL (Lanes 23 & 24), and albumin-predominant (lanes 27-29) complexes. B) Western blot and densitometric analysis showed that Levels of ApoL6 were significantly increased (p = 0.02, Fisher’s exact test) in 7 plasma samples of human ST-elevation AMI patients (P1, P3, P5, and P6 (upper panels); and P7, P8, and P9 (lower panels), ranging from 6 to 22 folds, as compared to the controls (C1 and C2 (upper panels); and C3, C4, and C5 (lower panels), respectively). Of note that these two WB membranes were probed with an anti-ApoL6 antibody [3] and then an anti-CRP antibody. The 2 lower membranes were duplicate of the upper membranes stained with Ponceau S for loading and quality validation.
Discussion
Previously we have shown that ApoL1 was present in the HDL fraction [6]. Our current results suggest a previously undescribed in vivo link between STEMI and ApoL6. ApoL6 is a pivotal component of the regulated cell death (RCD) pathways and likely plays an important role during coronary arterial plaque rupture. Although ApoL6 was detectable in plasma samples of control and STEMI patients, qualitatively and quantitatively assessed levels of ApoL6 in STEMI patients were substantially higher. The identification of increased ApoL6 in the sera of the majority of STEMI patients prior to reperfusion is highly suggestive of the activation of apoptotic pathways in ruptured plaque. However, activation of the 2 RCD pathways within ischaemic myocardium is also likely but cannot be distinguished in this study. Activation of ApoL6 and CRP in patients is consistent with the known inflammatory nature of coronary artery disease. Our observations suggest that activation of inflammatory apoptosis and/or necroptosis, in part triggered by ApoL6, is common in the early, pre-reperfusion phase of STEMI, and that ApoL6 could serve as a biomarker and a potential therapeutic target specific for inflammatory apoptotic and/or necroptotic activity.
Competing Interests
None.
Acknowledgements
This work was supported, in part, by the Robert S. Flinn Foundation (to WL).
Abbreviations
ApoL1: Apolipoprotein L1
CRP: C-Reactive Protein
MI: Myocardial Infarction
Article Info
Article Type
Research ArticlePublication history
Received: Wed 24, Jun 2020Accepted: Fri 07, Aug 2020
Published: Mon 24, Aug 2020
Copyright
© 2023 Chien-An Andy Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.04.10
Author Info
Chien-An Andy Hu Siqin Zhaorigetu W Sean Davidson Warren Laskey
Corresponding Author
Chien-An Andy HuDepartment of Biochemistry and Molecular Biology, University of New Mexico School of Medicine, Albuquerque, New Mexico, USA
Figures & Tables
References
- M A DeWood, J Spores, R Notske, L T Mouser, R Burroughs et al. (1980) Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 303: 897-902. [Crossref]
- Aloke V Finn, Masataka Nakano, Jagat Narula, Frank D Kolodgie, Renu Virmani (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30: 1282-1292. [Crossref]
- Siqin Zhaorigetu, Zhaoqing Yang, Ian Toma, Timothy A McCaffrey, Chien-An A Hu (2011) Apolipoprotein L6, induced in atherosclerotic lesions, promotes apoptosis and blocks Beclin 1-dependent autophagy in atherosclerotic cells. J Biol Chem 286: 27389-27398. [Crossref]
- Isabella Murphy, Guanghua Wan, Shulin Fu, Yulan Liu, Yinsheng Qiu et al. (2020) ApoL6 induces dichotomous cell death phenotype involving both apoptosis and necroptosis in cancer cells. Clin Oncol Res. DOI: 10.31487/j.COR.2020.07.12
- Chien-An A. Hu, Warren Laskey, Shulin Fu, Yinsheng Qiu, Yulan Liu et al. (2020) Apoptotic cellular models in cancer therapeutics. Clin Oncol Res. DOI: 10.31487/j.COR.2020.07.13
- Scott M Gordon, Jingyuan Deng, L Jason Lu, W Sean Davidson (2010) Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography J Proteome Res 9: 5239-5349. [Crossref]
- Guanghua Wan, Siqin Zhaorigetu, Zhihe Liu, Ramesh Kaini, Zeyu Jiang et al. (2008) Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 283: 21540-21549.
