Aggressive Adult Pancreatoblastoma with Early Recurrence: A Case Report
A B S T R A C T
Background: Pancreatoblastoma (PB) is a rare malignant epithelial tumor of the pancreas, mainly occurring in pediatric population. Pancreatoblastoma of the adult is extremely rare and usually shows a more aggressive biological behavior and a worse prognosis.
Case Presentation: The patient was a 54-year-old man admitted for abdominal pain and weight loss. A computed tomography (CT) scan showed a 12 cm heterogeneous hypoattenuating mass in the pancreatic head. An endoscopic ultrasound-guided biopsy suggested the diagnosis of pancreatoblastoma (PB). The patient underwent a pancreaticoduodenectomy (PD) combined with limited liver resections for intraoperative finding of multiple liver metastasis. Pathological findings confirmed the diagnosis of PB metastatic to the liver. The tumor recurred in the liver 5 months after surgery and the patient succumbed due to tumor dissemination 8 months after initial diagnosis.
Conclusion: Adult pancreatoblastoma is a rare pancreatic neoplasm with malignant behavior. The aim of this manuscript is to report our experience with pancreatoblastoma in the adult and to contribute improving knowledge about the clinical behavior of this rare and aggressive malignancy.
Keywords
Pancreatoblastoma, adult, pancreatic neoplasm, pancreatectomy
Introduction
Pancreatoblastoma (PB) is a rare malignant epithelial tumor of the pancreas, accounting for less than 1% of pancreatic neoplasms and with an annual incidence of approximately 0.004 per 100,000 people [1, 2]. First described by Becker et al. in 1957, as “infantile pancreatic carcinoma”, was subsequently renamed “pancreatoblastoma” by Horie et al. in 1977 for its morphologic similarity to fetal pancreatic tissue [3, 4]. Pancreatoblastoma mainly affects pediatric population, accounting for 25% of pancreatic neoplasms occurring in the first decade of life [5]. In contrast, adult pancreatoblastoma is extremely rare and usually more aggressive. Since Palosaari et al. described the first case of adult pancreatoblastoma in 1986, only 74 cases have been reported in literature so far [6]. Surgical resection is the treatment of choice, but there is no consensus on the best management of adult patients with pancreatoblastoma, since, because of its rarity, the clinical practice mainly relies on isolated case reports or small series reported in literature.
Here we report the case of a 54-year-old man diagnosed with pancreatoblastoma who underwent surgical resection, with intraoperative finding of multiple liver metastasis and recurred to the liver 5 months after surgery. The present report aims to contribute improving the existing clinical knowledge about the management of adult pancreatoblastoma.
Case Presentation
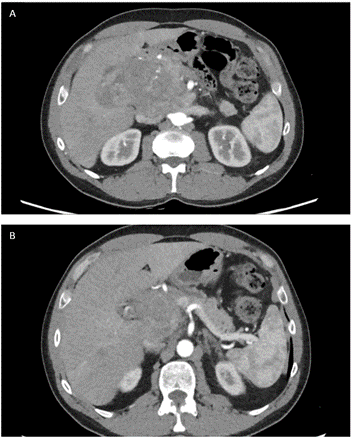
A 54-year-old man was admitted to our hospital due to abdominal pain and progressive weight loss (10 kgs in 2 months). His past medical history included no relevant information other than a previous surgery for varicocele repair in the childhood. A chest- abdomen computed tomography scan (CT- scan) showed a heterogeneous hypoattenuating mass measuring approximately 12 cm in the head of the pancreas with partially ill-defined and lobulated margins. The lesion shaped the confluence of the splenic and superior mesenteric veins but without signs of infiltration. The pancreatic contour blended with the lesion, causing dilatation of the main pancreatic duct (Figure 1). The laboratory workup, including tumor markers, was unremarkable, alfa- fetoprotein (AFP) was 276 (ng/mL).
Figure 1: A & B) CT- scan showed a heterogeneous hypoattenuating mass measuring approximately 12 cm in the head of the pancreas with partially ill-defined and lobulated margins. The lesion shaped the confluence of the splenic and superior mesenteric veins but without signs of infiltration.
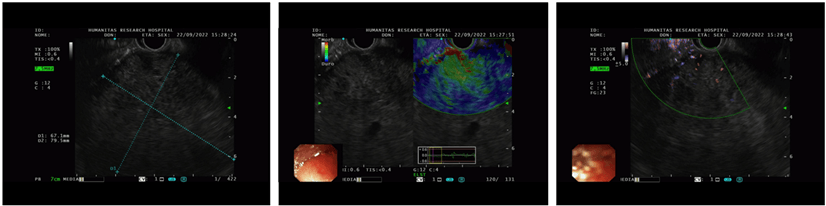
An endoscopic ultrasound (EUS) was performed, confirming the presence of a heterogeneous hypoechoic mass without a clear cleavage plan from the pancreas, and a core- biopsy was taken (Figure 2). Histological examination revealed sheets of neoplastic cells with rare acinar formation and focal swirling of the cells suggestive of squamoid nests. A panel of immunohistochemical stains was performed and the neoplastic cells focally labeled with cytokeratin 19 (CK19), alfa- fetoprotein (AFP) and beta-catenin. Immunolabeling with trypsin and chymotrypsin highlighted the areas with acinar differentiation. The morphology and immunohistochemical labeling pattern suggested the diagnosis of pancreatoblastoma.
Figure 2: An endoscopic ultrasound (EUS) was performed, confirming the presence of a heterogeneous hypoechoic mass without a clear cleavage plan from the pancreas.
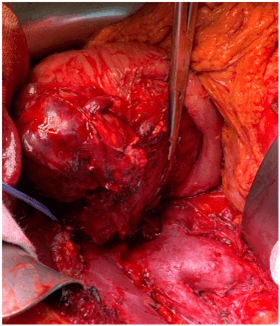
The case was discussed in the multidisciplinary tumor board meeting and the patient was scheduled for a pancreaticoduodenectomy (PD). Pre- operative laboratory workup showed an alfa- fetoprotein (AFP) of 689 ng/mL. Intraoperatively, a liver lesion on the Glissonian’s surface highly suspicious for metastasis was detected in segment 2 (S 2). An intra-operative liver ultrasound (IOUS) with contrast enhancement was carried out for staging, confirming the presence of an isoechoic heterogeneous lesion in S 2 and two additional subcentrimentric lesions in segment 3 (S 3). Considering the resectability of the liver lesions, a pilorus- preserving pancreaticoduodenectomy combined with IOUS- guided S 2 resection and a limited resection of S 3 was performed (Figure 3). The post- operative course was uneventful, and the patient was discharge on post- operative day 9.
Figure 3: The intraoperative findings showed that the tumor was capsular and was in contact with the confluence of the splenic and superior mesenteric veins; however, obvious infiltration of these vessels was not observed.
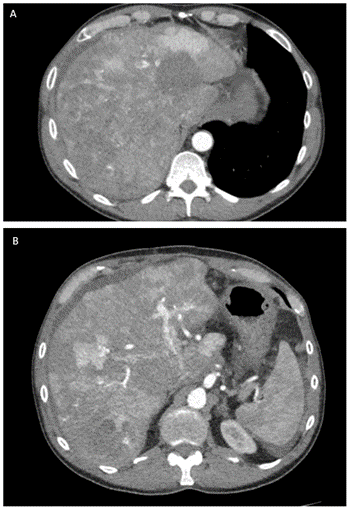
Figure 4: A & B) CT- scan 5 months after surgery showed multiple liver lesions consistent with liver metastases.
Final pathology showed a 15 cm tumor of the head of the pancreas with negative margins and 12 lymph nodes negative for tumor involvement with vascular but no perineural invasion. Both histology and immunohistochemical labeling (CK AE1/AE3, CK5/6, CK19, EMA - Epithelial membrane antigen, sinaptofisin, cromogranin, INSM1- Insulinoma-associated protein 1, cl10- Anti-Folate receptor beta, AFP, Hepatocyte, beta-catenin) confirmed the diagnosis of pancreatoblastoma. The pathology examination also confirmed the presence of three liver metastases measuring, respectively, 1.4, 1.1 and 0.8 cm. Final pathological staging was pT3 N0 M+ (liver). The patient did not undergo adjuvant chemotherapy and started the follow-up. At the first follow-up 5 months after surgery, the CT- scan showed multiple large liver lesions consistent with metastases (Figure 4).
The patient was then scheduled to start systemic treatment with Doxorubicin and Cisplatinum but his conditions rapidly deteriorated and two weeks later he was admitted for bowel obstruction, treated conservatively. A new CT- scan showed disease progression in the liver and peritoneal carcinosis. The patient was then referred to palliative care and died 8 months after surgery.
Discussion
Pancreatoblastoma (PB) is a rare malignant epithelial tumor of the pancreas, mainly occurring in pediatric population. Pancreatoblastoma of the adult is extremely rare and usually shows a more aggressive biological behavior and a worse prognosis. To the best of our knowledge, since first described by Palosaari et al. in 1986, only 74 cases of adult PB have been reported in literature [6]. The etiology is unknown and even if most cases are sporadic, few adult pancreatoblastomas have been described in the setting of familial adenomatous polyposis (FAP) and associated with Beckwith- Wiedemann syndrome [2, 7-9].
Adult pancreatoblastoma more frequently occurs in the head of the pancreas, followed by tail and body [10]. The diagnosis might be challenging because of its resemblance with other pancreatic neoplasms and non- specific symptoms at presentation, more frequently represented by abdominal pain and weight loss [10]. At imaging PB usually presents as large and well-defined heterogeneous mass and there are no significant differences in the imaging findings of adult versus pediatric patients [10, 11].
Enhancement is a common feature on contrast-enhanced computed tomography images and might be present on magnetic resonance imaging (MRI). At MRI, PB shows low to intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images [12]. Tumor markers, including Ca 19.9 and CEA, are usually in the normal range and AFP is rarely elevated in adult pancreatoblastoma [7]. Microscopically, pancreatoblastomas are composed of cellular well-delineated lobules separated by dense fibrous band. PB predominantly show acinar differentiation; other cell lines like ductal, neuroendocrine and mesenchymal differentiation are less commonly identified but may be present [13]. The characteristic histological feature of pancreatoblastoma is the squamoid nests, which can help in distinguishing PB tumor cells with acinar differentiation from acinar cell carcinoma or solid-pseudopapillary neoplasm [14].
Pancreatoblastomas typically express trypsin, chymotrypsin, lipase, and BCL10. Squamoid nests may be positive for EMA, AE1/AE3 or CD10 and nuclear and cytoplasmic expression of β-catenin may be present [1, 7]. To date, the staging of pancreatoblastoma follows the TNM classification of carcinoma of the exocrine pancreas [15]. Surgical resection is usually treatment of choice, but there is no consensus on the best management of adult patients with pancreatoblastoma and the role of adjuvant chemotherapy or radiotherapy is not clear yet, based on the very small number of patients treated. Here we reported the case of an aggressive adult pancreatoblastoma in 54-year-old man who underwent surgical resection, with intraoperative finding of multiple liver metastases. The patient had liver recurrence 5 months after surgery and succumbed due to tumor dissemination 8 months after initial diagnosis.
Of the 74 cases of adult pancretoblastoma reported in the literature, outcome information is available for 57 patients. The median overall survival for adult patients with pancreatoblastoma reported in literature is 15 months (range, 1– 108 months) [10]. Among the cases described in literature, metastasis and/or local invasion was found at diagnosis or operation, in 59 % of patients [1]. Liver is usually the most common site of metastasis, followed by lymph nodes and lungs [1, 2, 7, 10, 17, 18]. Chest wall, breast, bone, and brain metastases are described in literature, but they are exceptionally rare [1, 7, 19].
The biological reasons of this aggressive behavior in the adults are still unclear. Recently, molecular aberration in the fibroblast growth factor receptor (FGFR) signalling pathway, including somatic FGFR1 mutation, FGFR2 gene rearrangement, have been identified with RNA sequencing [20]. Further studies are needed to better understand the biological features of this rare tumor and to identify potential molecular targets to select patients that might benefit from systemic treatment strategies.
Conclusion
Adult pancreatoblastoma is a rare pancreatic neoplasm with malignant behavior. The prognosis is dismal due to advanced stage at presentation and disease recurrence. Diagnosis might be challenging because of its resemblance with other pancreatic neoplasms and non- specific symptoms at presentation. Surgical resection is usually the treatment of choice, but there is no consensus on the best management of adult patients with pancreatoblastoma and the current clinical management mostly relies on case report and small case series. Further data are needed to improve the knowledge about the clinical behavior of this rare and aggressive malignancy.
Disclosures
None.
Informed Consent
Written informed consent was obtained by the patient, and no IRB was required since all patient information was removed and de-identified.
Article Info
Article Type
Case ReportPublication history
Received: Thu 20, Jul 2023Accepted: Mon 28, Aug 2023
Published: Mon 02, Oct 2023
Copyright
© 2023 Martina Nebbia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2023.05.02
Author Info
Martina Nebbia Silvia Uccella Silvia Carrara Silvia Bozzarelli Francesca Gavazzi Cristina Ridolfi Giovanni Capretti Gennaro Nappo Michele Pagnanelli Alessandro Zerbi
Corresponding Author
Martina NebbiaPancreatic Surgery Unit, Humanitas Research Hospital IRCCS, Via Manzoni, Rozzano, Milan, Italy
Figures & Tables
References
1. Salman B, Brat G,
Yoon YS, Hruban RH, Singhi AD et al. (2013) The diagnosis and surgical
treatment of pancreatoblastoma in adults: a case series and review of the
literature. J Gastrointest Surg 17: 2153-2161. [Crossref]
2. Klimstra DS, Wenig
BM, Adair CF, Heffess CS (1995) Pancreatoblastoma. A clinicopathologic study
and review of the literature. Am J Surg Pathol 19: 1371-1389. [Crossref]
3. BECKER WF (1957)
Pancreatoduodenectomy for carcinoma of the pancreas in an infant; report of a
case. Ann Surg 145: 864-870. [Crossref]
4. Horie A, Yano Y,
Kotoo Y, Miwa A (1977)) Morphogenesis of pancrea- toblastoma, infantile
carcinoma of the pancreas: report of two cases. Cancer 39: 247-254. [Crossref]
5. Klimstra DS (2007)
Nonductal neoplasms of the pancreas. Mod Pathol 20 Suppl 1: 94-112. [Crossref]
6. Palosaari D,
Clayton F, Seaman J (1986) Pancreatoblastoma in an adult. Arch Pathol Lab
Med 110: 650-652. [Crossref]
7. Zouros E, Manatakis
DK, Delis SG, Agalianos C, Triantopoulou C et al. (2015) Adult
pancreatoblastoma: A case report and review of the literature. Oncol Lett
9: 2293-2298. [Crossref]
8. Abraham SC, Wu TT,
Klimstra DS, Finn LS, Lee JH et al. (2001) Distinctive molecular genetic
alterations in sporadic and familial adenomatous polyposis-associated
pancreatoblastomas : frequent alterations in the APC/beta-catenin pathway and
chromosome 11p. Am J Pathol 159: 1619-1627. [Crossref]
9. Drut R, Jones MC
(1988) Congenital pancreatoblastoma in Beckwith-Wiedemann syndrome: an emerging
association. Pediatr Pathol 8: 331-339. [Crossref]
10. Omiyale AO (2015)
Clinicopathological review of pancreatoblastoma in adults. Gland Surg 4:
322-328. [Crossref]
11. Rosebrook JL, Glickman
JN, Mortele KJ (2005) Pancreatoblastoma in an adult woman: sonography, CT, and
dynamic gadolinium-enhanced MRI features. AJR Am J Roentgenol 184:
S78-S81. [Crossref]
12. Montemarano H,
Lonergan GJ, Bulas DI, Selby DM (2000) Pancreatoblastoma: imaging findings in
10 patients and review of the literature. Radiology 214: 476-482. [Crossref]
13. Terino M, Plotkin
E, Karagozian R (2018) Pancreatoblastoma: an Atypical Presentation and a
Literature Review. J Gastrointest Cancer 49: 361-364. [Crossref]
14. Wood LD, Klimstra
DS (2014) Pathology and genetics of pancreatic neoplasms with acinar
differentiation. Semin Diagn Pathol 31: 491-497. [Crossref]
15. Nagtegaal ID, Odze
RD, Klimstra D, Paradis V, Rugge M et al. (2020) The 2019 WHO classification of
tumours of the digestive system. Histopathology 76: 182-188. [Crossref]
16. Balasundaram C,
Luthra M, Chavalitdhamrong D, Chow J, Khan H (2012) Pancreatoblastoma: a rare
tumor still evolving in clinical presentation and histology. JOP 13:
301-303. [Crossref]
17. Hammer STG, Owens
SR (2013) Pancreatoblastoma: a rare, adult pancreatic tumor with many faces. Arch
Pathol Lab Med 137: 1224-1226. [Crossref]
18. Pitman MB, Faquin
WC (2004) The fine-needle aspiration biopsy cytology of pancreatoblastoma. Diagn
Cytopathol 31: 402-406. [Crossref]
19. Elghawy O, Wang JS, Whitehair RM, Grosh W, Kindwall Keller TL (2021) Successful treatment of metastatic pancreatoblastoma in an adult with autologous hematopoietic cell transplant. Pancreatology 21: 188-191. [Crossref]
20. Berger AK, Mughal SS, Allgäuer M, Springfeld C, Hackert T et al. (2020) Metastatic adult pancreatoblastoma: Multimodal treatment and molecular characterization of a very rare disease. Pancreatology 20: 425-432. [Crossref]
