A Case of Double Primary Carcinoma was Accidentally Found During Operation: Hepatocellular Carcinoma and Appendiceal Mucinous Adenocarcinomas
A B S T R A C T
Background: Synchronous double primary cancer of hepatocellular carcinoma (HCC) and appendiceal mucinous adenocarcinomas has not been reported. Liver metastasis of digestive tract tumors is so common that it is easy to ignore the existence of double primary cancer in the digestive system. The appendiceal malignant tumor itself is easy to be misdiagnosed or missed. For a better understanding of HCC and appendiceal mucinous adenocarcinomas, the information on our recent case has been summarized.
Case Presentation: A 58-year-old man was admitted with repeated upper abdominal pain for one week. Imaging examination and liver biopsy pathology were diagnosed as hepatocellular carcinoma. Colonoscopy showed no obvious organic lesions in the terminal ileum and the whole large intestine. We had anatomical resection of the S5 and S6 segments of the liver. At the same time, two suspicious lesions in the right liver were ablated by microwave. But at the end of the operation, we accidentally found a hard mass about 3 cm × 3 cm in size at the end of the appendix. And the rapid pathological results showed appendiceal adenocarcinoma, so we had a right hemicolectomy. So far, the diagnosis of the patient was changed to synchronous double primary cancer consisting of HCC and appendiceal mucinous adenocarcinomas.
Conclusion: It is the first time we have reported a case of synchronous double primary hepatic cancer consisting of HCC and appendiceal mucinous adenocarcinomas in the world. Due to the rarity and unspecific clinical features, it is extremely challenging to be diagnosed preoperatively. It requires a more comprehensive understanding of the patient's medical history, careful physical examination, and comprehensive exploration of the abdominal cavity during the operation to avoid missed diagnosis. Patients with HCC and appendiceal mucinous adenocarcinomas are generally associated with poor prognosis. It needs we put forward new countermeasures for the new question.
Keywords
Synchronous double primary cancer, hepatocellular carcinoma, appendiceal mucinous adenocarcinomas
Background
Appendiceal cancer is a rare and frequently aggressive malignancy that includes a variety of histological subtypes. Current estimates suggest that the incidence of appendix tumors is approximately 0.12-0.97 per 100,000 population [1, 2]. The incidence of appendiceal adenocarcinoma is about 6%-20% in all appendiceal malignant tumors [3, 4]. Appendiceal mucinous adenocarcinoma is only one type of appendiceal adenocarcinoma, including signet ring and non-mucinous adenocarcinoma. Appendiceal tumors are often misdiagnosed or missed because of nonspecific clinical manifestations.
Liver cancer is predicted to be the sixth most commonly diagnosed cancer and the fourth leading cause of cancer worldwide in 2018, with about 841,000 new cases and 782,000 deaths annually. Hepatocellular carcinoma (HCC) comprises 75%-85% of liver cancer [5]. The main risk factors for HCC are chronic infection with hepatitis B virus (HBV) in China [6]. We often see cases of gastrointestinal tumor metastasis to the liver in clinical work, for example, liver metastases from colon cancer. But synchronous double primary cancer consisting of HCC and appendiceal mucinous adenocarcinoma is rarely encountered in clinical practice. No synchronous double primary cancer of HCC and appendiceal mucinous adenocarcinoma cases have been reported. During the operation, we accidentally found the 58-year-old patient diagnosed with synchronous double primary cancer with appendiceal mucinous adenocarcinomas and HCC. Here we report the case of synchronous double primary hepatic cancer consisting of HCC and appendiceal mucinous adenocarcinomas for the first time globally.
Case Presentation
I Chief Complaints
A 58-year-old man was admitted with the main complaint of repeated upper abdominal pain for one week.
II History of Present Illness
The patient complained of intermittent epigastric pain, paroxysmal distending pain, and no radiating pain one week ago. There was no fever, chills, nausea, vomiting, exhaustion, or defecation. Abdominal pain can be relieved by itself, but repeated attacks and the degree of abdominal pain gradually aggravate.
III History of Past Illness
The patient underwent endoscopic choledocholithotomy for bile duct stones in our hospital on March 2, 2017. He was also diagnosed with hepatitis B for over 20 years and cirrhosis in 2017. In January 2019, a colonoscopy showed multiple colorectal polyps, and endoscopic polypectomy was performed. Gastroscopy showed reflux esophagitis, hiatal hernia, and erosive gastritis. He has suffered from hypertension for 15 years and has been taking antihypertensive medication for a long time with good blood pressure control. The patient had no history of coronary heart disease, diabetes mellitus, or allergies.
IV Personal and Family History
The patient's father died of cerebrovascular accident for many years. Close relatives of the patient did not suffer from the same disease as the patient.
V Physical Examination Upon Admission
The patient's temperature was 36.6 °C, heart rate was 78 bpm, respiratory rate was 19 breaths per min, and blood pressure was 128/90 mmHg. The abdominal muscles are soft without tenderness and rebound pain. No significant positive signs on physical examination.
VI Laboratory Examinations
The white cell count was 7.76 × 109/L, and haemoglobin was 142 g/L. The serum ferritin level was less than 1.0 µg/L (normal range: 15-200μg/L). AFP 64.09μg/L, CEA7.49μg/L. Coagulation was abnormal: APTT 53.3s, FIB 5.01g/L. HBV DNA was below the detection limit. The function of the liver, kidney, and electrolyte were normal. The electrocardiogram was normal.
VII Imaging Examinations
Chest X-ray showed thickening of left lower pleura and no abnormality of heart and lung. B-ultrasound results showed liver cirrhosis and multiple solid space-occupying lesions in liver S5 and S6 segments, which did not exclude the possibility of liver cancer, and cholestasis in the gallbladder. The spleen is large, and there was no obvious abnormality in the pancreas. The results of contrast-enhanced CT in the upper abdomen showed that the lesions in the S5 and S6 segments of the liver were considered to be hepatocellular carcinoma with the possibility of sub-focus formation, liver cirrhosis, portal hypertension, collateral circulation formation; multiple cysts in the liver. The results of contrast-enhanced MRI using the hepatocyte-specific contrast agent Gd-EOB-DTPA were consistent (Figure 1). A liver biopsy showed moderately differentiated hepatocellular carcinoma. The results of the gastroscopy showed that: Reflux esophagitis (LA-A); hiatal hernia; chronic nonatrophic gastritis, mainly gastric antrum. The colonoscopy results showed no apparent organic lesions in the mucosa of the terminal ileum and the whole large intestine, including Internal hemorrhoids.
Figure 1: The result of contrast-enhanced MRI using the hepatocyte-specific contrast agent Gd-EOB-DTPA shows liver S5, and S6 segment lesions, consider hepatocellular carcinoma with sub-focus formation.
Treatment
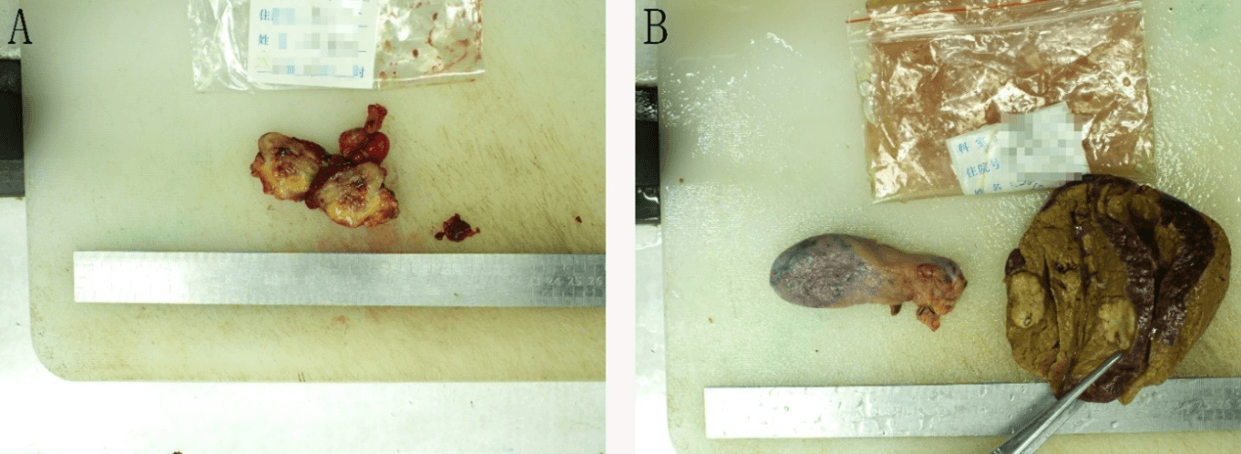
The patient underwent right hepatic S5 and S6 segments resection, microwave ablation of liver puncture, and radical right hemicolectomy. During the operation, there were about 200ml of old hematocele in the abdominal cavity, no abnormality in the stomach, small intestine, spleen, and pancreas, no obvious metastatic cancer nodules in the omentum and pelvic cavity, three cancer foci in the liver protruding from the surface of the liver, all located at S5 and S6 segment of liver, about 2 cm × 3 cm, hard in texture, pale in section, and clear in boundary, so we had S5 and S6 hepatectomy. At the same time, two suspicious lesions in the right liver were ablated by microwave. After the above operation, a 3 cm × 3 cm hard mass was unexpectedly found at the end of the appendix, which adhered to the peritoneum (Figure 2). The surface of the mass was ruptured with a little bleeding and scab. During the operation, the appendix was removed and sent for rapid pathological examination, which indicated that: (mass around the appendix) adenocarcinoma infiltration was found in the appendix wall. Radical right hemicolectomy was added.
Figure 2: A shows a 3cm × 3cm hard mass at the end of the appendix. B shows the S6 segment of the liver where the tumor was removed and the gallbladder.
Final Diagnosis
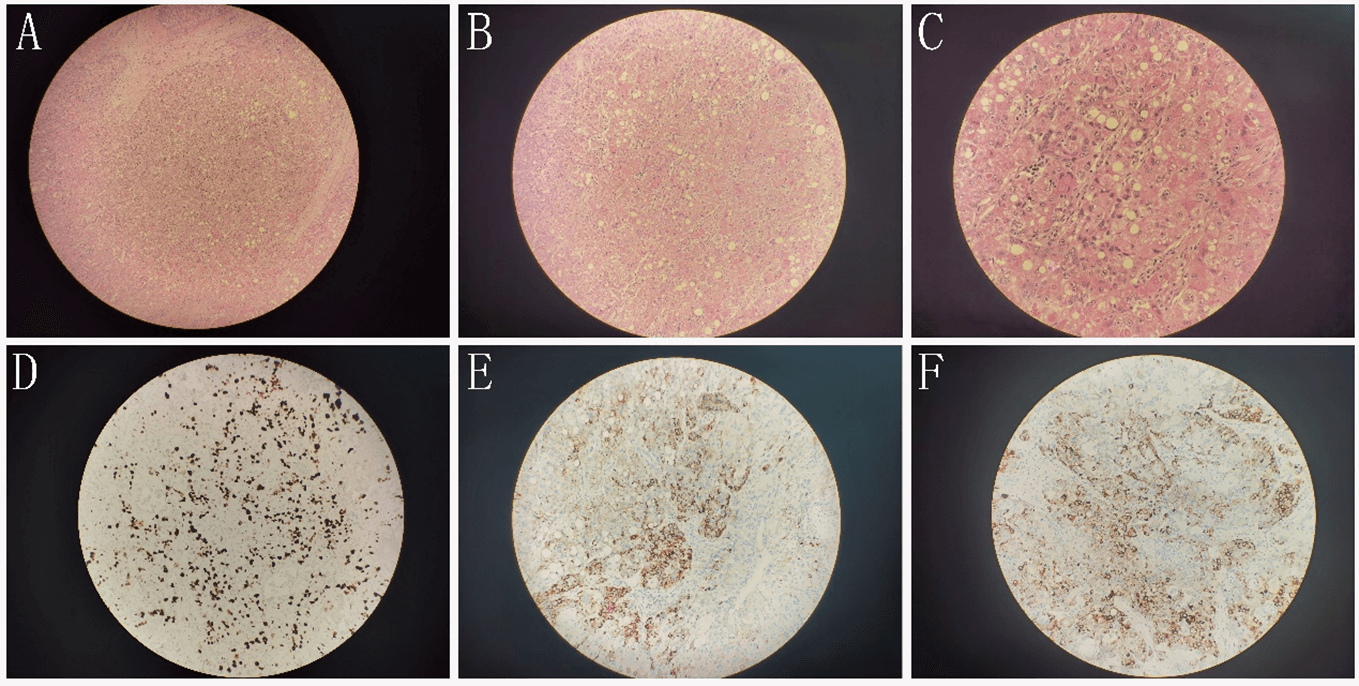
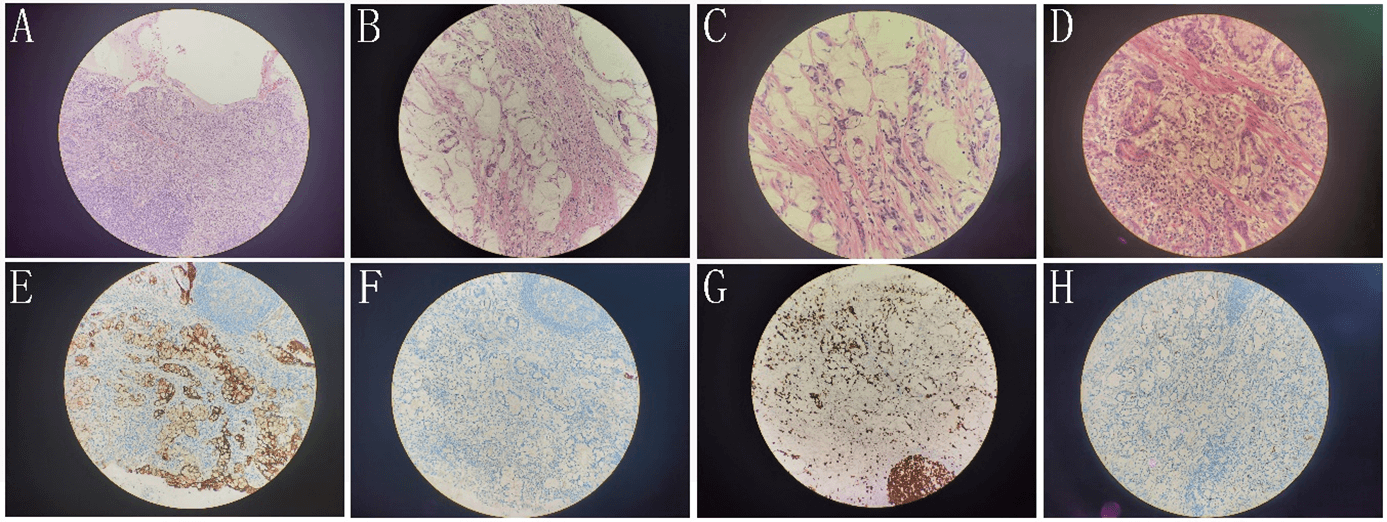
The pathological results of the patients after the operation showed that right hepatocellular carcinoma. There are tumor specimens. One is poorly differentiated and has local necrosis. Immunohistochemical results: CD34 (sinusoidal endothelium +), Ki-67 (hot spot area about 30%), p53 (about 50% intensity+), HBsAg (+), EGFR (+) (Figure 3). The other one is moderately differentiated, and surrounding liver tissue showed cirrhosis. The liver capsule and the cutting edge did not involve cancer. Mucinous adenocarcinoma of the appendix invaded the whole wall of the appendix. The tumor cells showed mucus in and out of the cell and mucus lake formation. Some cells were signet ring, and a stromal vascular tumor thrombus was formed. Immunohistochemical results: CK7 (-), CK20 (+), Satb2 (+), BRAF (-), MLH1 (+), PMS2 (+), p53 (about 5% strength +), Ki-67 (about 20% ~ 30%+), CD56 (-), syn (-), CgA (-) (Figure 4). The surrounding appendiceal mucosal glands were locally serrated hyperplasia, and no cancer cells were involved in the appendectomy margin. (right colon) chronic inflammation of intestinal mucosa, no residual cancer. Both sides of the intestinal stump, anastomotic-attached intestinal tissue, free small intestine, free colon, and omental tissue were negative. One of the 14 lymph nodes around the intestine had metastatic carcinoma.
Figure 3: A) (100x), B) (200x), and C) (400x) are the HE staining of liver cancer. E) (200x), F) (200x) and G) (200x) are immunohistochemical staining of Ki67(hot spot area about 30%+), HBsAg (+) and EGFR (+), respectively.
Figure 4: A) (100x), B) (200x), C) (400x), and D) (400x) are the HE staining of appendix mucinous adenocarcinoma. The tumor cells showed mucus in and out of the cell and mucus lake formation. Some cells were signet ring and a stromal vascular tumor thrombus was formed. E-H) are immunohistochemical staining of CD20(+) (200x), CK7(-) (200x), Ki67(about 20%~30%+) (200x), and Syn (-) (200x), respectively.
The final diagnosis by histopathology was appendiceal mucinous adenocarcinomas (T3N1M0 IIIB) and hepatocellular carcinoma (T2N0M0 II). The patients also had liver cirrhosis, reflux esophagitis, hiatal hernia, gastritis, and hypertension (a very high-risk group).
Outcome and Follow-Up
The patient recovered and was discharged after 13 days of hospitalization. He returned to the hospital for reexamination on January 17, 2021. The liver function was normal. Postoperative whole abdominal enhanced CT showed the changes after hepatectomy, microwave ablation, and right hemicolectomy. No obvious lesions were found in the liver.
Discussion and Conclusion
Appendiceal mucinous neoplasms (AMNs) are rare tumors accounting for less than 1% of all cancers. AMNs include a heterogeneous group of diseases with varying malignant potential, as reflected by different classification systems [7]. Initially, Woodruff and McDonald classified AMNs into two categories: benign cysts and cystadenocarcinoma [8]. Since then, due to different disputes, its content has been changing. In 2009, Pai and Longacre classified AMNs into four categories: mucinous adenoma, low-grade mucinous neoplasm with low or high risk of recurrence, and mucinous adenocarcinoma [9]. The incidence of appendiceal adenocarcinoma is about 6% in all appendiceal malignant tumors [3]. Appendiceal adenocarcinomas are rare in clinical work and can be mucinous, non-mucinous, or signet-ring subtypes [10]. However, the rarity of the condition, a lack of nonspecific clinical manifestations, specific tumor biomarkers, and the limited information provided by imaging make it difficult to confirm the correct diagnosis before surgery for doctors [11]. An acute appendicitis-like presentation with right lower quadrant pain secondary to distention of the appendix by mucin is the most common clinical presentation in early-stage disease [7].
Carr et al. reported that 32% of patients with appendiceal neoplasms received a preoperative diagnosis of acute appendicitis, while 23% were incidentally diagnosed [12]. Our patient had no symptoms or signs of the right lower abdomen, and the colonoscopy showed no noticeable organic lesions in the terminal ileum and the whole large intestine. Based on the above, we naturally think there was no problem with the appendix and didn't do any imaging of the right lower abdomen for the patient. We focused all our attention on liver cancer because the pathological result of the liver biopsy of the patient was HCC.
Liver cancer is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide in 2018. HCC comprises 75%-85% of liver cancer [5]. In China, primary liver cancer is the fourth most common malignancy and the second leading cause of tumor-related death, thereby posing a significant threat to the life and health of the chinese people. The main pathological subtypes of primary liver cancer are HCC, intrahepatic cholangiocarcinoma (ICC), and HCC-ICC. HCC is the most common primary liver cancer, accounting for 85–90% of all cases [13]. Our patient has HBV and liver cirrhosis. The serum AFP (64.09μg/L) was high but not more than 400μg/L. B-ultrasound-enhanced CT and enhanced MR all showed multiple lesions in the S5 and S6 segments of the liver and the largest intrahepatic nodules with a diameter of >2 cm. The clinical diagnosis of HCC can be established. Finally, a liver biopsy confirmed HCC. During the operation, we found three lesions in segment VI of the liver, which protruded from the surface of the liver. We had an anatomical resection of segment IV of the liver. At the same time, two suspicious lesions in the right liver detected by intraoperative ultrasound were ablated by microwave. A large amount of warm normal saline was used to wash the abdominal cavity.
In the course of HCC resection, we unexpectedly found a hard mass with a size of 3 cm × 3 cm at the end of the appendix, which adhered to the peritoneum. The surface of the appendix was ruptured, and there was a little bleeding and blood scab. The appendix was removed during the operation and sent for rapid pathological examination. The result is appendiceal mucinous adenocarcinomas. So far, synchronous double primary cancer consisting of HCC and appendiceal mucinous adenocarcinomas has been diagnosed. Multiple primary cancers refer to two or more kinds of primary malignant tumors occurring simultaneously or successively in the same or different organs. Most of the multiple primary cancers are double primary malignant tumors. The diagnosis interval of multiple malignant tumors is synchronous multiple primary cancer within 6 months, and metachronous multiple primary cancer is more than 6 months. In clinical practice, we often encounter cases of liver metastasis from colorectal cancer [14]. However, there are few cases of liver metastases from appendiceal malignancies. We searched the relevant data and found no cases of synchronous double primary cancer consisting of HCC and appendiceal mucinous adenocarcinomas. Only one case of triple synchronous malignant tumors of the colon, appendix, and liver was reported by Shen Guoliang et al. [15]. But this patient's appendix tumor is carcinoid and not appendiceal mucinous adenocarcinomas.
In patients with appendiceal adenocarcinoma, the rate of metastatic disease to regional lymph nodes ranges from 20% to 67%. Because of this substantial risk, nonmetastatic adenocarcinoma confined to the appendix should be treated with the right hemicolectomy [16]. During the operation, the fast pathological results of the patient showed appendiceal adenocarcinoma, so we had a right hemicolectomy. Postoperative pathology showed that the appendix tumor was larger than 2 cm, and one of 14 lymph nodes had metastasis. The patient needed systemic chemotherapy. 5-fluorouracil–based systemic chemotherapy (similar to that used for colorectal adenocarcinoma) is typically recommended for patients with high-grade peritoneal disease or nodal metastases [16]. But the optimal chemotherapeutic drug regimen continues to be investigated. One disadvantage of the case is that he did not receive hyperthermic intraperitoneal chemotherapy (HIPEC). Shaib et al. evaluated the role of HIPEC in patients with primary appendiceal mucinous adenocarcinoma in a retrospective analysis. The median OS was 77 months for patients who received HIPEC compared with 25 months for patients who did not (p<0.001) [17].
From this case, we can learn the following points. First, we should improve the preoperative examination, such as whole abdominal enhanced CT or PET-CT, to avoid missed diagnosis. Careful exploration is needed to avoid missing lesions during the operation. Second, double primary cancer is rare, but it should be considered. Third, the treatment of synchronous double primary hepatic cancer consisting of HCC and appendiceal mucinous adenocarcinomas is challenging. We need to standardize our treatment of it.
Conclusion
We report the case of synchronous double primary hepatic cancer consisting of HCC and appendiceal mucinous adenocarcinomas for the first time in the world. The case expands our knowledge. Collecting and summarizing the information of the case will help doctors to diagnose and treat this disease.
Acknowledgements
Thanks to the colleagues in the imaging department for helping to read the image results.
Author Contributions
Yi Zhou reviewed the relevant literature, collected the patient’s clinical data, and participated in the drafting of the manuscript. Peng Gao, Xian-Cheng Zeng, and Guo-Wei Zhang performed the surgery; Bo-Hao analysed and diagnosed the pathology. Hai-Liang Li designed the report, analysed the data, and revised the paper; all authors have read and approved the final version of this manuscript.
Funding
Funding by Science and Technology Projects in Guangzhou(202102021064) and Doctoral workstation foundation of Guangdong Second Provincial General hospital(2021BSG8011).
Ethics Approval and Consent to Participate
Not applicable as this is a case report, not a clinical study.
Consent for Publication
Written informed consent for the publication of patient clinical details and clinical images was obtained from the patient.
Competing Interests
The authors declared there were no competing interests.
Abbreviations
HCC: Hepatocellular Carcinoma
HBV: Hepatitis B Virus
AFP: Alpha Fetopro
CEA: Carcinoembryonic Antigen
APTT: Activated Partial Thromboplastin Time
FIB: Fibrinogen
CT: Computed Tomography
MRI: Magnetic Resonance Imaging
Gd-EOB-DTPA: Gadolinium-Ethoxybenzyl-Diethylene-Triamine Pentaacetic Acid
S5/6: Segment 5/6
EGFR: Epidermal Growth Factor Receptor
AMNs: Appendiceal Mucinous Neoplasms
ICC: Intrahepatic Cholangiocarcinoma
BRAF: Recombinant B-Raf Proto Oncogene Serine/Threonine Protein Kinase
MLH1: MutL Homolog-1
Article Info
Article Type
Case ReportPublication history
Received: Wed 23, Aug 2023Accepted: Mon 11, Sep 2023
Published: Mon 30, Oct 2023
Copyright
© 2023 Hai-Liang Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2023.05.04
Author Info
Yi Zhou Peng Gao Xian-Cheng Zeng Guo-Wei Zhang Bo-Hao He Hai-Liang Li
Corresponding Author
Hai-Liang LiDepartment of General Surgery, Guangdong Second Provincial General Hospital, Guangzhou, Guangdong Province, China
Figures & Tables

References
1. Marmor S, Portschy
PR, Tuttle TM, Virnig BA (2015) The rise in appendiceal cancer incidence:
2000-2009. J Gastrointest Surg 19:
743-750. [Crossref]
2. McCusker ME, Cote
TR, Clegg LX, Sobin LH (2002) Primary malignant neoplasms of the appendix: a
population-based study from the surveillance, epidemiology and end-results
program, 1973-1998. Cancer 94:
3307-3312. [Crossref]
3. Shang J, Ruan LT,
Dang Y, Wang YY, Song Y et al. (2016) Contrast-enhanced ultrasound improves
accurate identification of appendiceal mucinous adenocarcinoma in an old
patient: A case report. Medicine
(Baltimore) 95: e4637. [Crossref]
4. Smeenk RM, van
Velthuysen MLF, Verwaal VJ, Zoetmulder FAN (2008) Appendiceal neoplasms and pseudomyxoma
peritonei: a population based study. Eur
J Surg Oncol 34: 196-201. [Crossref]
5. Bray F, Ferlay J,
Soerjomataram I, Siegel RL, Torre LA et al. (2018) Global cancer statistics
2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin 68:
394-424. [Crossref]
6. Chen W, Zheng R,
Baade PD, Zhang S, Zeng H et al. Cancer statistics in China, 2015. CA Cancer J Clin 66: 115-132. [Crossref]
7. Shaib WL, Assi R,
Shamseddine A, Alese OB, Staley C 3rd et al. Appendiceal Mucinous Neoplasms:
Diagnosis and Management. Oncologist 22:
1107-1116. [Crossref]
8. Rouzbahman M,
Chetty R (2014) Mucinous tumours of appendix and ovary: an overview and
evaluation of current practice. J Clin
Pathol 67: 193-197. [Crossref]
9. Pai RK, Beck AH,
Norton JA, Longacre TA (2009) Appendiceal mucinous neoplasms: clinicopathologic
study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol 33: 1425-1439. [Crossref]
10. Assarzadegan N,
Montgomery E (2021) What is New in 2019 World Health Organization (WHO)
Classification of Tumors of the Digestive System: Review of Selected Updates on
Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med 145: 664-677. [Crossref]
11. Zhang W, Tan C, Xu
M, Wu X (2020) Primary appendiceal mucinous neoplasm: Gynecological
manifestations, management, and prognosis. Gynecol
Oncol 156: 357-362. [Crossref]
12. Carr NJ, McCarthy
WF, Sobin LH Epithelial noncarcinoid tumors and tumor-like lesions of the
appendix. A clinicopathologic study of 184 patients with a multivariate
analysis of prognostic factors. Cancer 75:
757-768. [Crossref]
13. Zhou J, Sun H, Wang
Z, Cong W, Wang J et al (2020) Guidelines for the Diagnosis and Treatment of
Hepatocellular Carcinoma (2019 Edition). Liver
Cancer 9: 682-720. [Crossref]
14. Engstrand J,
Nilsson H, Stromberg C, Jonas E, Freedman J (2018) Colorectal cancer liver
metastases - a population-based study on incidence, management and survival. BMC Cancer 18: 78. [Crossref]
15. Guoliang S,
Dongsheng H (2013) Triple synchronous malignant tumors of colon, appendix and
liver: A case report with literature review. Pak J Med Sci 29: 237-238. [Crossref]
16. Glasgow SC, Gaertner W, Stewart D, Davids J, Alavi K et al. The American Society of Colon and Rectal Surgeons, Clinical Practice Guidelines for the Management of Appendiceal Neoplasms. Dis Colon Rectum 62: 1425-1438. [Crossref]
17. Shaib WL, Martin LK, Choi M, Chen Z, Krishna K et al (2015) Hyperthermic Intraperitoneal Chemotherapy Following Cytoreductive Surgery Improves Outcome in Patients With Primary Appendiceal Mucinous Adenocarcinoma: A Pooled Analysis From Three Tertiary Care Centers. Oncologist 20: 907-914. [Crossref]
