Unusual Case of Pseudomyxoma Peritonei of a Mature Teratoma in Adult Men
A B S T R A C T
Pseudomyxoma peritonei (PMP) is a rare disease usually originating from appendiceal neoplasm and its incidence is 1-2 cases per million. Extra-appendicular origin is less common and is related to ovary, urachus, stomach, colon and pancreas cancer. A few cases of mature ovarian teratoma associated with PMP have been described. We report an extremely rare case of a man treated for a mature teratoma recurrence as a PMP. A 57-year-old man with multiple surgeries for mature teratoma excision as a newborn was addressed to our institution. At the age of 55, a surgical exploration found a low-grade PMP. The patient was asymptomatic, and surveillance was performed. Nine months later, a sub-complete cytoreductive surgery (CRS) with Mitomycin C HIPEC (hyperthermic intraperitoneal chemotherapy) was performed. One year later, the residual mass volume was treated by a new CRS with Mitomycin C HIPEC. After a two-year follow-up patient has no recurrence. PMP extra-appendiceal origin in less than 10%. This is the first reported case of PMP originating as a mature teratoma recurrence in a man. A few cases describe PMP from ovarian teratoma in women. Despite PMP’s different origins, gold standard treatment still remains CRS and HIPEC.
Keywords
Teratoma, pseudomyxoma, HIPEC
Background
Pseudomyxoma peritonei (PMP) is a very rare peritoneal malignancy with an estimated incidence of one to three per million people annually [1]. It is characterized by the accumulation of mucinous ascites into the abdominal cavity and it usually originates from appendiceal perforated mucinous neoplasm while less frequently it is secondary to ovary, urachus, stomach, colon and pancreas cancer [2-8]. PMP arising from mature ovarian teratomas are described in the literature [9, 10]. The origin is related to the mucinous epithelial component of the teratoma, as showed by histology and immunochemistry (CK20+/CK7-) [8].
Clinical presentation can vary from completely asymptomatic patients who are diagnosed incidentally during surgery or imaging to patients having abdominal distention due to abundant ascites, pain, loss of weight and digestive disorders. The gold standard treatment for PMP is, complete cytoreductive surgery (CRS), associated with hyperthermic intraperitoneal chemotherapy (HIPEC) [11, 12]. Here we report the first case of PMP arising from mature teratoma in an adult man.
Case Presentation
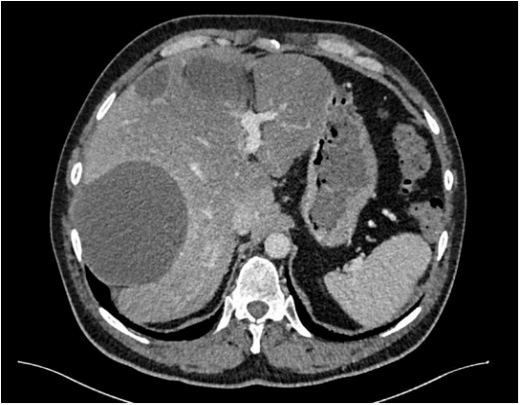
A 57-year-old man with a history of five surgeries for mature teratoma excision as a newborn, was addressed to our institution. At the age of 55, due to constipation, an abdominal ultrasound was performed showing multiples cystic abdominal lesions and a teratoma recurrence was suspected. Surgical exploration found a mucinous collection into the right upper quadrant, between the right liver and the diaphragm. Biopsies were performed and pathology confirmed a low-grade PMP with acellular mucin pool. Immunochemistry was negative for MUC4 and cytokeratin. Tumor markers (Carcinoembryonic antigen-CEA, cancer antigen-CA 19-9, cancer antigen-CA 125) were negatives. Explorations were completed postoperatively with a colonoscopy and a gastroscopy without any relevant findings. The patient was asymptomatic and a wait and watch attitude was performed. Nine months later, a CT scan showed a growing up pseudomyxoma with multiples collections, the biggest of 11 cm into the right sub-phrenic space (Figure 1).
Figure 1: Preoperative CT scan showing inter-hepatico-diaphragmatic cyst.
Surgical exploration found a plastic peritonitis due to multiple adhesions between small bowel and colon, a disseminated carcinomatosis with a peritoneal cancer index (PCI) of 17 and 9 regions attempted. A sub-complete CRS with Mitomycin C (15 mg/m2) HIPEC for 90 minutes at 43°C was performed. The inter-hepato-diaphragmatic cyst was not treated to avoid extra morbidity in a patient who had right colectomy, subtotal gastrectomy and liver metastasectomy and had almost 2 liters bleeding. Histology found multiple localisations of mature teratoma associated with acellular mucin. MUC4 was not expressed as well as CK7 and CK20. No carcinoma or immature teratoma was found.
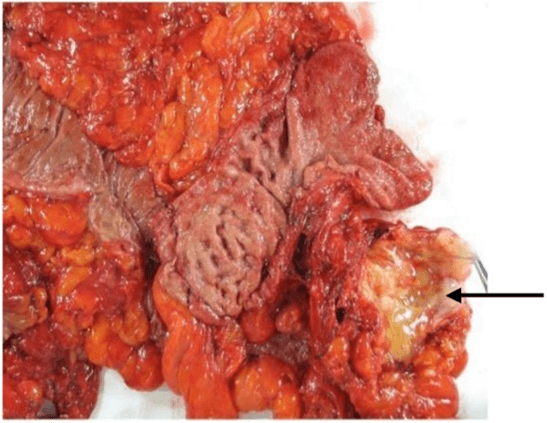
One year later, residual mass volume increased and the patient accepted a new CRS with hepatic VI-VII bisegmentectomy, partial right diaphragm resection and Mitomycin C HIPEC (15 mg/m2) for 90 minutes at 43°C. During abdominal exploration, there was no recurrence of the pseudomyxoma treated a year earlier. Histology found mature teratoma on all resected specimens again (Figure 2). After a two-year follow-up patient has no recurrence.
Figure 2: Macroscopic view of one of the specimens (pericolic carcinomatosis nodule marked by an arrow).
Discussion
Teratoma is the commonest congenital tumor. The most frequent site is sacrococcygeal, while some others are mediastinum, head and neck, oropharynx, pericardium, and retroperitoneum. In newborn, prognosis is favourable with 80-100% survival, reported after excisional surgery [13, 14]. They are commonly evocated when evaluating a mass in the female pelvis. In the abdominal extragonadal site, they are quite rare. They can affect both sexes and can present at any age. Most frequently found in ovary and testis, they arise from totipotent cells and contain derivatives of one to all three germ cell layers: the ectoderm, endoderm and mesoderm [15, 16]. Teratomas are classified as mature or immature, depending on the degree of differentiation of their components. PMP is most commonly associated with the intra-abdominal spread of an appendiceal mucinous neoplasm. Pseudomyxoma peritonei from ovarian malignancy was first described by R. Werth in 1884 [17]. In 1901, E. Frankel reported the first case of appendicular mucocele with abdominal mucinous dissemination [18]. PMP associated with mucinous tumors, arising from mature ovarian teratoma are rare and a few cases are reported in the literature in women [5, 9, 10, 19, 20].
To our knowledge, this is the first case of PMP associated with mature teratoma in a man. Our patient showed a CK7-/CK20- phenotype. He developed a teratoma as a newborn and had multiple recurrences after surgery. Mature teratomas are the most common of the germ cell tumors. Usually, they occur during reproductive years and are composed of different types of tissues, solid and cystic. Mucinous tumors arising in ovarian mature cystic teratomas are thought to arise from the gut elements of the teratoma [20]. Due to the small number of extra-appendicular origin of PMP, treatment still remains CRS/HIPEC as in appendicular origin. No differences in prognosis and clinical-pathological features have been reported in the literature comparing appendicular or extra-appendicular origin pseudomyxoma. As reported by Vang et al., pseudomyxoma associated with ovarian mucinous neoplasm is more common in teratoma associated mucinous tumors with CK7-/CK20+ phenotype expression [21].
Two-stage cytoreductive surgery is not a common technique and a few cases are reported in the literature. Even in expert centres, huge pseudomyxoma still remains unresectable in near 30%. Sgarbura et al. recently reported a small series of eight patients with a huge PMP treated by two-stage CRS + HIPEC [22]. The median time interval between two surgeries was 4 months. They reported a complete pathologic response during the second surgery. According to this study, two-stage surgery should be taken into consideration in patients with huge PMP (PCI >30) who are not eligible to complete cytoreduction during the first surgery.
In conclusion, when PMP is found in a male or female patient with a history of operated mature teratoma it should be taken into consideration than extra-appendicular origin arising from teratoma recurrence and it should be treated by CRS/HIPEC.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Sat 15, Aug 2020Accepted: Sat 29, Aug 2020
Published: Mon 05, Oct 2020
Copyright
© 2023 Cecilia Ceribelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CRSS.2020.02.04
Author Info
Cecilia Ceribelli M. Gomes David A. Ayav J. Raft A. Leroux F. Marchal
Corresponding Author
Cecilia CeribelliDepartment of Surgery, Institut de Cancérologie de Lorraine-Alexis Vautrin, Université de Lorraine, Vandoeuvres-lès-Nancy, Nancy, France
Figures & Tables
References
- Syed Ali Rizvi, Wajahat Syed, Ravi Shergill (2018) Approach to pseudomyxoma peritonei. World J Gastrointest Surg 10: 49-56. [Crossref]
- X B Li, Z H Ji, Y L Lin (2019) Research progress of CRS + HIPEC in treatment of pseudomyxoma peritonei. Med Recapitulate 25: 915-921.
- N Ansari, K Chandrakumaran, S Dayal, F Mohamed, T D Cecil et al. (2016) Cytoreductive surgery andhyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelialtumours. Eur J Surg Oncol 42: 1035-1041. [Crossref]
- R M Smeenk, M L F van Velthuysen, V J Verwaal, F A N Zoetmulder (2008) Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34: 196-201. [Crossref]
- Brigitte M Ronnett, Jeffrey D Seidman (2003) Mucinous tumors arising in ovarian mature cysticteratomas: relationship to the clinical syndrome of pseudomyxoma peritonei. Am J Surg Pathol 27: 650-657. [Crossref]
- P J G Goldstein, J Cabanas, R G da Silva, P H Sugarbaker (2006) Pseudomyxomaperitonei arising from colonic polyps. Eur J Surg Oncol 32: 764-766. [Crossref]
- Wen Wang, Lingbin Meng, Eric Crespo, Jeffrey Adams, Manoucher Manoucheri (2019) Gelatinous Abdomen: A Rare Case of Pseudomyxoma Peritonei Arising from Metastatic Gastric Adenocarcinoma. Cureus 11: e4666. [Crossref]
- N Pranesh, L P Menasce, M S Wilson, S T O'Dwyer (2005) Pseudomyxoma peritonei: unusual origin from an ovarian mature cystic teratoma. J Clin Pathol 58: 1115-1117. [Crossref]
- K R Lee, R E Scully (2000) Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol 24: 1447-1464. [Crossref]
- Colin J R Stewart, Tetsuya Tsukamoto, Bridget Cooke, Yee C Leung, Ian G Hammond (2006) Ovarian mucinous tumour arising in mature cystic teratoma and associated with pseudomyxoma peritonei: report of two cases and comparison with ovarian involvement by low‐grade appendiceal mucinous tumour. Pathology 38: 534-538. [Crossref]
- Norman J Carr, Jenny Finch, Ian Charles Ilesley, Kandiah Chandrakumaran, Faheez Mohamed et al. (2012) Pathology and prognosis in pseudomyxoma peritonei: a review of 274 cases. J Clin Pathol 65: 919-923. [Crossref]
- Patrick L Wagner, Frances Austin, Mazen Zenati, Aaron Jaech, Arun Mavanur et al. (2016) Oncologic risk stratification following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for appendiceal carcinomatosis. Ann Surg Oncol 23: 1587-1593. [Crossref]
- N M Marina, B Cushing, R Giller, L Cohen, S J Lauer et al. (1999) Complete surgical excision is effective treatment for children with immature teratoma with or without malignant elements: a Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol 17: 2137-2143. [Crossref]
- S N Huddart, J R Mann, K Robinson, F Raafat, J Imeson et al. (2003) Sacrococcygeal teratomas: the UK Children’s Cancer Study Group’s experience. Pediatr Surg Int 19: 47-51. [Crossref]
- D Tapper, E E Lack (1983) Teratomas in infancy and childhood. A 54-year experience at the Children’s Hospital Medical Center. Ann Surg 198: 398-410. [Crossref]
- Teruko Ueno, Yumiko Oishi Tanaka, Michio Nagata, Hajime Tsunoda, Izumi Anno et al. (2004) Spectrum of germ cell tumors: from head to toe. Radiographics 24: 387-404. [Crossref]
- R Werth (1884) Klinische und anatomische Untersuchungen zur Lehre von den Bauchgeschwülsten und der Laparotomie: II-Pseudomyxoma Peritonei. Archiv Für Gynaekologie 24: 103-121.
- E Frankel (1901) Uher das sogenanute pseudomyxoma peritonei. Med Wochenschr 48: 965-970.
- Jong Ha Hwang, Kyeong A So, Gayatri Modi, Jae Kwan Lee, Nak Woo Lee et al. (2009) Borderline-like mucinous tumor arising in mature cystic teratoma of the ovary associated with pseudomyxoma peritonei. Int J Gynecol Pathol 28: 376-380. [Crossref]
- Yoshimasa Gohda, Rei Noguchi, Tomoko Horie, Toru Igari, Harumi Nakamura et al. (2016) Pseudomyxoma peritonei of a mature ovarian teratoma caused by mismatch repair deficiency in a patient with Lynch syndrome: a case report. BMC Med Genet 17: 94. [Crossref]
- Russell Vang, Allen M Gown, Chengquan Zhao, Todd S Barry, Christina Isacson et al. (2007) Ovarian mucinous tumors associated with mature cystic teratomas: morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered as secondary tumors in the ovary. Am J Surg Pathol 31: 854-869. [Crossref]
- Olivia Sgarbura, Mohammed Al Hosni, Andrea Petruzziello, Rodrigo Figueroa, Lakhdar Khellaf et al. (2020) Complete pathologic response after two-stage cytoreductive surgery with HIPEC for bulky pseudomyxoma peritonei: proof of concept. Int J Hyperthermia 37: 585-591. [Crossref]
