Topical and Capsular RM Diabetes Wound Cure for Treatment of Diabetic Foot Ulcer
A B S T R A C T
Background: Despite the prevalence and quality of life impact of diabetic foot ulcer (DFU), novel topical and oral therapy standard-of-care remain an important research priority. To date, the lack of available data has made it difficult to assess the efficacy of topical and capsular RM diabetes wound cure for DFUs.
Case Presentation: We report the case of a 59-year-old Nigerian woman P2+0 with 2 living children, a known diabetes mellitus patient since 2006 and has been compliant with her antidiabetic medications, who presented with complaint of right foot ulcer. We also report the case of a 69-year-old Nigerian man that presented with DFU. The latter is a known diabetic and hypertensive diagnosed 15 years prior to presentation. For the first case, both topical and capsular RM wound cure were applied twice daily over an area of the ulcer for 8 weeks. For the second case, only RM diabetes wound cure capsule applied twice daily was received for 8 weeks. Efficacy was analysed by lesion measurements and photographs to determine overall response rate (ORR), and complete response (CR). All doses were well tolerated, and potentially efficacious. At 8 weeks across all dose levels, subjects achieved 100.0% ORR and 50.0% CR.
Conclusion: Application of topical and ingestion of the oral capsule of RM diabetes wound cure appear safe and well tolerated with some reduction in lesion pain. Some greater levels of lesion stabilization was observed in the subjects, and this demonstrated great clinical benefit in foot ulcers. A randomized, placebo-controlled trial to confirm these findings is recommended.
Keywords
Capsule, diabetic foot, powder, randomized, ulcer, wound infections
Introduction
Diabetes mellitus (DM) is a collection of metabolic and pathophysiological disorders manifested with high glucose levels in the blood due to the inability of β-pancreatic cells to secrete an adequate amount of insulin or insensitivity of insulin towards receptor to oxidize blood glucose [1]. In cases where a person who has been diagnosed with Type 1 or Type 2 diabetes mellitus sustains an injury and the treatment of the damage is complicated and prolonged, the injury is referred to as a diabetic ulcer and if the foot or the hand is involved as the case may be, a diabetic foot ulcer (DFU) or a diabetic hand ulcer respectively. The presence of many proliferating macrophages at the injury site for an extended period causes damage and breakdown of the skin and surrounding tissues, with the development of and worsening of diabetic wound [1].
Diabetic foot ulcers (DFUs) are the most common chronic wounds, and they constitute a serious complication of diabetes mellitus because of its association with pain, disability, and poor quality of life [2, 3]. Their prevalence has been increasing significantly over the past decade, consuming scarce health care resources [4, 5]. In diabetic foot ulcers, wound treatments should aim to alleviate symptoms, promote healing, and avoid adverse outcomes, especially lower extremity amputation. Topical antimicrobial therapy has been used on diabetic foot ulcers, either as a treatment for clinically infected wounds, or to prevent infection in clinically uninfected wounds [6]. The recent Cochrane review has recommended that investigators should undertake properly designed studies on the effects of topical antimicrobial treatments for both the prevention and the treatment of infection in DFUs and its wound healing [6].
There are no published studies on the use of topical RM diabetes wound cure powder (produced by Regina Mundi Diabetes, Uzoagba, Nigeria) for the treatment of DFUs. RM diabetes wound cure powder is a natural preparation from the Vernonia amygdalina A1, B1 and C, Cucurbita, Crongromena iatifolia leaves and Gnetum africanum. It is also available as capsule, known as RM diabetes, wound cure capsule. RM wound cure powder has been approved under Listing status by the National Agency for Food and Drug Administration and Control (NAFDAC) with NAFDAC registration number of A7-1564L as safe for use while allowing data generation on efficacy through clinical trial. This research aimed at providing such scientific evidence for the safety and efficacy of this herbal preparation. This, in conjunction with its broad-spectrum exfoliative mechanism-of-action, provided the basis for the investigation of RM diabetes wound cure, as a topical treatment for DFUs.
The potential advantages of topical interventions over systemic therapy include ease of administration, decreased distress, reduced toxicity and the creation of a feeling of patient-directed control [7]. Based on anecdotal reports, it is anticipated that RM diabetes wound cure powder would facilitate the transport of dia-D powder through the skin into the dermis, providing a continuous anti-diabetogenic and anti-inflammatory dose of RM diabetes wound cure powder to DFU over the dosing interval with limited systemic exposure. It is also anticipated, based on anecdotal reports that the application of the powder to DFU and the surrounding skin would be well tolerated, allowing for repeat self-administration to large areas of the body, if necessary. We present a case each of two DFU patients that received either both topical and capsular RM wound cure or only capsular RM wound cure.
Case Presentation
Case 1
The patient is a 59-year-old Nigerian woman P2+0 with 2 living children, a known diabetes mellitus patient since 2006, who presented with complaint of right foot ulcer. She sustained the wound since 2004 and had undergone several treatments including two different skin grafting. She has been regular with her anti diabetic medication.
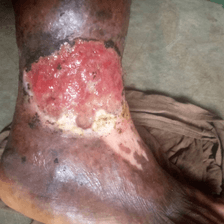
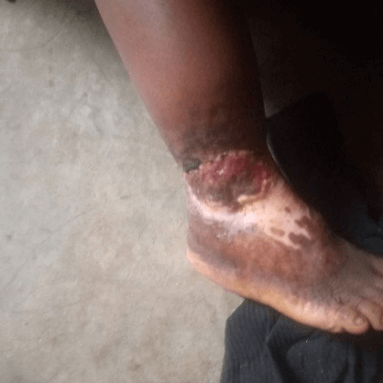
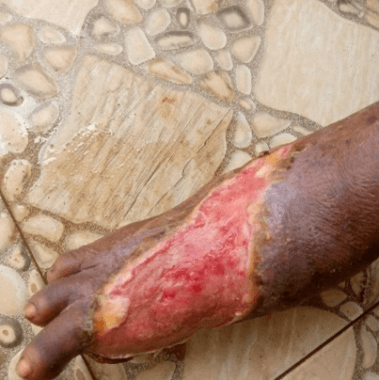
Physical examination (Figure 1) revealed an otherwise healthy looking lady with normal gait. Right lower limb examination revealed a shallow foot ulcer, about 10cm × 8cm in dimension, with a spanning exposed wound. The left lower limb was normal. The case was placed under observation for the effects of the treatment. There was no nausea and vomiting episodes. At 2 weeks of treatment, there was marked progress (Figure 2) and at 8 weeks of treatment, the patient achieved 100.0% ORR and 50.0% CR (Figure 3).
Figure 1: The diabetic foot before therapy for case 1.
Figure 2: The diabetic foot at 2 weeks of therapy for case 1.
Case 2
The patient is a 69-year-old Nigerian man that presented 7 months earlier with diabetic foot ulcer. He is a known diabetic and hypertensive diagnosed about 15 years prior to presentation. He has since been regular with his drugs including hydrochlorothiazide, atorvastatin, metformin etc. He had disarticulation of his right big and the second toes. His last Fasting blood sugar was 115mg/dl, with a blood pressure of 140/90mmHg.
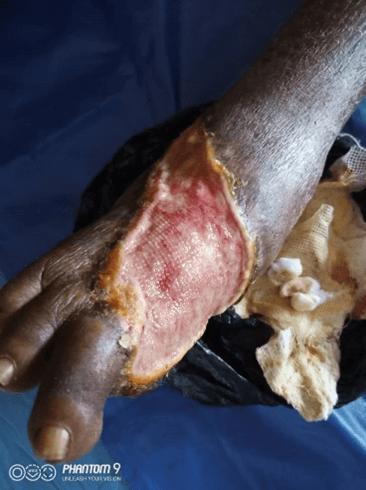
Physical examination revealed an otherwise healthy looking man with abnormal gait. Right lower limb examination revealed a shallow foot ulcer, about 8cm × 8cm in dimension (Figure 4). The left lower limb was normal. The case was under observation for the effects of the treatment. There was no nausea and vomiting episodes. The serum electrolyte, urea and creatinine, liver function test and full blood count were normal at base line for case 1 and case 2. At 2 weeks of treatment, there was marked progress (Figure 5) and at 8 weeks of treatment, the patient achieved 100.0% ORR and 50.0% CR.
Figure 3: The diabetic foot at 8 weeks of therapy for case 1.
Figure 4: The diabetic foot before therapy for case 2.
Figure 5: The diabetic foot at 2 weeks of therapy for case 2.
Discussion
Infected diabetic foot ulcer (IDFU) is a public health concern and the principal cause of non-traumatic limb amputation. Despite high burden of DFU in Africa, there are limited published data on DFU in most West African countries, including Nigeria [7, 8]. Ulceration frequently heralds foot infection in diabetic patients, with peripheral vascular disease, peripheral neuropathy, visual impairment and immunological disturbances also playing influential roles. Infection blights the healing process and worsens the condition of patients with DFU. This might lead to great disability, septicemia and death if not promptly and appropriately managed [8]. In a recent systematic review and meta-analysis on the worldwide burden of DFU in West African sub-region, the authors concluded that the dearth of data encumbers the strategies and novel interventions for treatment and prevention of DFUs and infections [9]. This has given impetus to the present case report.
Despite the prevalence and quality of life impact of DFU, novel topical and oral therapy standard-of-care remains an important research priority. To date, however, the lack of available data has made it difficult to assess the efficacy of topical RM diabetes wound cure powder and capsule for the treatment diabetic foot ulcers. The topical RM diabetes wound cure powder and capsule have shown to be potentially useful in cases of chronic diabetes mellitus patients. Their use could not be limited to treatment naïve individuals because the two patients involved in this case reports were patients who have been diagnosed for more than a decade old.
This new drug regimen demonstrates that treatment should continue for at least 8 weeks for therapeutic benefits. The potential safety could be inferred by the normal findings found in serum electrolytes, urea, creatinine, liver function test and full blood count parameters seen at base line and following therapy. The oral administration appears well tolerated since there was no reported nausea and vomiting episodes in the cases presented. The potential differences in the effects of the two forms of therapy (topical and oral applications) will require clinical trial demonstrations.
Conclusion
Application of topical RM diabetes wound cure powder and capsular RM diabetes wound cure appear safe and well tolerated with some reduction in lesion pain. Measurable levels of lesion stabilization were observed in the subjects over the study period. The results demonstrated the great potential clinical benefit of the topical and oral application of RM diabetes wound cure in foot ulcers among diabetics. A randomized, placebo-controlled trial to confirm these findings is recommended.
Acknowledgement
The study was coordinated by the Effective Care Research Unit at Nnamdi Azikiwe University, Awka, Nigeria. The authors appreciate the help of the staff of Nnamdi Azikiwe University Teaching Hospital (NAUTH), Nnewi, Nigeria and participants involved in the case report. Publication of these results should not be considered an endorsement of any product used in this study by the Nnamdi Azikiwe University or any of the organizations where the authors are affiliated.
Author Contributions
GUE, CME, EUN, CBO, IDA, and COO designed the study and carried out the procedures for this project, while other authors (AAO, ICA, EAE, AAO, KCI, CGC and CUU) carried out the procedures for this project including study design, execution, and acquisition of data, analysis and interpretation. GUE supervised the overall conceptual design, implementation of the project, and revision of the manuscript. All authors agreed on the journal to which the article was submitted. All authors were involved in the writing and substantial revision of this manuscript. All authors contributed to the critical review and final approval of the manuscript. All the authors read, approved the final manuscript and agreed to be accountable for all aspects of the work.
Funding
The research was funded by the Regina Mundi Herbal Products Nigeria Limited, Uzoagba, Ikeduru, Imo State, Nigeria and the researchers. The funders had no role in the design, conduct, analysis, interpretation, or write up of the study.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the authors on reasonable request.
Ethical Approval
Not applicable.
Consent for Publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing Interests
None.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Wed 10, Aug 2022Accepted: Mon 22, Aug 2022
Published: Fri 09, Sep 2022
Copyright
© 2023 George Uchenna Eleje. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2022.09.04
Author Info
George Uchenna Eleje Chidiebele Malachy Ezeude Celestine Ogonna Oguejiofor Charlotte Blanche Oguejiofor Iffiyeosuo Dennis Ake Patrick Maxwell Uche Ifeoma Clara Ajuba Chinyelu Uchenna Ufoaroh Ekene Agatha Emeka Arinze Anthony Onwuegbuna Chisom God’swill Chigbo Kindness Chidi Irikannu Ekeuda Uchenna Nwankwo
Corresponding Author
George Uchenna ElejeEffective Care Research Unit, Department of Obstetrics and Gynaecology, Nnamdi Azikiwe University, Nnewi campus, Nigeria
Figures & Tables

References
1. Mariadoss AVA, Sivakumar AS, Lee CH,
Kim SJ (2022) Diabetes mellitus and diabetic foot ulcer: Etiology, biochemical
and molecular based treatment strategies via gene and nanotherapy. Biomed
Pharmacother 151: 113134. [Crossref]
2. Oliveira D, Borges A, Saavedra MJ,
Borges F, Simões M (2022) Screening of Natural Molecules as Adjuvants to
Topical Antibiotics to Treat Staphylococcus aureus from
Diabetic Foot Ulcer Infections. Antibiotics (Basel) 11: 620. [Crossref]
3. Ku HC, Liang YJ (2018)
Incretin-based therapy for diabetic ulcers: from bench to bedside. Expert
Opin Investig Drugs 27: 989-996. [Crossref]
4. Hüsers J, Moelleken M, Richter ML,
Przysucha M, Malihi L et al. (2022) An Image Based Object Recognition System
for Wound Detection and Classification of Diabetic Foot and Venous Leg Ulcers. Stud
Health Technol Inform 294: 63-67. [Crossref]
5. Gebrekirstos LG, Abadi MT,
Gebremedhin MH, Lake EA, Wube TB (2022) Diabetic Foot Ulcer Among Adults
Attending Follow-Up Diabetes Clinics in Wolaita Zone, Southern Ethiopia: An
Unmatched, Case-Control Study. Curr Ther Res Clin Exp 96: 100673. [Crossref]
6. Dumville JC, Lipsky BA, Hoey C,
Cruciani M, Fiscon M et al. (2017) Topical antimicrobial agents for treating
foot ulcers in people with diabetes. Cochrane Database Syst Rev 6: CD011038.
[Crossref]
7. Ugwu E, Adeleye O, Gezawa I, Okpe I,
Enamino M et al. (2019) Burden of diabetic foot ulcer in Nigeria: Current
evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. World
J Diabetes 10: 200-211. [Crossref]
8. Adeyemo AT, Kolawole B, Rotimi VO, Aboderin AO (2021) Multicentre study of the burden of multidrug-resistant bacteria in the aetiology of infected diabetic foot ulcers. Afr J Lab Med 10: 1261. [Crossref]
9. Zhang P, Lu J, Jing Y, Tang S, Zhu D et al. (2017) Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 49: 106-116. [Crossref]
