The Supraclavicular Skin Temperature Response to Mild Cold Stimulation is Dependent on Ambient Temperature
The Supraclavicular Skin Temperature Response to Mild Cold Stimulation is Dependent on Ambient Temperature
A B S T R A C T
Purpose: This study investigated the basal activity, and cold-induced thermogenic response, of supraclavicular brown adipose tissue (BAT) under warm (23˚C) and cool (18˚C) ambient conditions using supraclavicular skin temperature as a measure of BAT activity. As a highly metabolic, heat-producing tissue, it has been hypothesised that under-active/dysfunctional BAT may underlie a pathological energy imbalance leading to obesity.
Methods: Five lean, healthy participants underwent infrared thermography (IRT) of supraclavicular BAT before, and during, mild cold exposure (single-hand immersion in cool water at 20˚C), once at 18˚C and once at 23˚C. Energy expenditure (EE) was measured simultaneously using indirect calorimetry, and mean skin temperature (TMSK) was calculated at 1-minute intervals in parallel to IRT using wireless data loggers.
Results: Following 30 minutes of hand cooling, supraclavicular skin temperature (TSCR) rose significantly from baseline at an ambient temperature of 23˚C (∆TSCR: 0.17 ± 0.03˚C, P < 0.01), and EE rose by 0.22 ± 0.02 kJ/min, P < 0.001. At an ambient room temperature of 18˚C, TSCR after hand cooling was similar to baseline, and EE remained unchanged. The TMSK response was indicative of a systemic vasoconstrictive response of similar magnitude in both warm and cool ambient temperatures.
Conclusions: At 18˚C in light clothing, BAT may already be maximally stimulated at baseline, and respond minimally to additional cold exposure. Ambient temperature is recognised as a determinant of glucose uptake in BAT. In this study, we show, that it also modulates the TSCR response to further localised cold-stimulation, indicating an effect on BAT thermogenesis.
Keywords
Brown adipose tissue, BAT, non-shivering thermogenesis, ambient temperature, obesity
Introduction
Following the recent discovery of functional, metabolically active brown adipose tissue (BAT) in humans, interest lies in elucidating the mechanisms underlying the BAT mediated non-shivering thermogenic (NST) component of the physiological response to acute cold [9, 10, 28, 29, 30]. It is well established that cold-induced activation of uncoupling protein (UCP)1 on the inner mitochondrial membrane of thermogenic brown adipocytes uncouples oxidative phosphorylation from the generation of ATP, and that the excess chemical energy is dissipated as heat [6]. As a result, fat depots containing an abundance of thermogenic UCP1 containing adipocytes generate heat and expend energy [3, 11]. It is estimated that cold-induced BAT activation could increase resting energy expenditure by at least 2.5-5% [27]. The most superficial thermogenic BAT depot in humans is found in the neck and upper thorax [9], and supraclavicular skin temperature has been used in conjunction with varying cold stimuli (e.g.: localised chest cooling, personalised whole body cooling protocols designed to achieve maximal NST) as a proxy measure of thermogenesis [15, 14 ,5, 16]. As a highly metabolic tissue, BAT has a high glucose requirement, and radio-labelled glucose uptake is often used as an indicator of BAT activity. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is frequently used for clinical purposes, and warming patients prior to scanning is recommended to reduce FDG uptake in BAT (which may obscure the region of clinical interest [26]. Retrospective studies of clinically indicated FDG-PET scans have identified that BAT is less frequently observed in individuals who have been subject to a range of pre-warming procedures and, prospective BAT dedicated studies confirm an acute BAT response to both warming and cooling [8, 25, 17, 31].
Furthermore, the primary substrates of BAT are lipids, and UCP1 activation is fatty acid-dependent [4]. Glucose uptake is, therefore, a crude measure of BAT activity, and when quantified as standard uptake values (SUV) on static FDG-PET-CT (e.g. SUVmean, SUVmax) may be considered semi-quantitative at best [20]. Importantly, these measures do not quantify thermogenic output. How exposure to warm ambient conditions before exposure to a cold stimulus affects the subsequent heat production from BAT, has not been explored. In this study, we used direct measurements of supraclavicular skin temperature to investigate the basal activity and cold-induced thermogenic response of supraclavicular brown adipose tissue (BAT) under warm (23˚C) and cool (18˚C) ambient conditions. We hypothesised that the basal activity of BAT would be lowest under warm conditions and that the response to mild cold exposure would, therefore, be greater.
Methods
The study was undertaken following the University of Nottingham School of Medicine Ethics Committee approval (Reference no: D052011) during the months of October and November. All participants gave written informed consent to take part, and the study conformed to the standards set by the Declaration of Helsinki 2008, in place at the time.
I Participants
All esterification reactions of CALB-catalyzed synthesis of ethyl butyrate were carried out in identical solvent-free systems, in a jacketed filter reactor, as it has been described previously [10]. In all cases, the method of initial velocities was followed, whereas the concentration of substrate butyric acid was ranged from 0.10 M up to 2.75 M. The use of butyric acid concentrations higher than 2.75 M would exceed the 20% of the whole esterification reaction mixtures applied in this work. A typical kinetic run comprised the addition of the reactants in the reactor, in that order: 28.5 ml anhydrous ethanol or anhydrous deuterated ethanol, the appropriate quantity of anhydrous butyric acid (e.g. 1.40 mL, i.e. 0.50 M), and 20 mg of CALB ([E]0 = 4.04 Μ), which initiated the reaction. Subsequently, the reactor was thermostated at 40oC and stirred continuously (100 rpm), whereas the formed water was maintained stable (0.01 v/v) by pervaporation under vacuum of 10 mbar [10]. The esterification reactions were followed up to the first 25 min, when the % of esterification was less than 5%; at that time the concentration of ethyl butyrate, as well as its yield were monitored. For this reason, aliquots were withdrawn at time intervals, diluted in anhydrous ethanol and analyzed by CG - FID chromatography, as previously [1, 10]. The kinetic runs were performed in triplicates and their mean values were considered; the standard deviations of triplicates were found less than ± 5%, in all cases. At the end of the esterification procedures the contained water was estimated by the coulometric method of Karl Fischer [1, 10]. The quantitative determinations concerning the % yields and the concentrations of the synthesized ethyl butyrate were performed by means of DANI MASTER GC Fast Flame ionization detector system whose the GC-profiles were analyzed and quantified as previously [1, 10].
II Cooling protocol
Participants attended the laboratory on two mornings, and the cooling protocol was undertaken at room temperatures of 18˚C and 23˚C. Measurements commenced after a minimum of 45 minutes’ stabilisation to the room temperature. After a 15 minute basal period, the left hand was immersed in a 9L bucket of cool water (20˚C) to the level of the ulnar styloid process. Water temperature was checked every 5 minutes, ice water added if it had risen by more than 0.2˚C until temperature was restored to 20˚C, and equal volumes of water removed to maintain the water depth to the level of the ulnar styloid process.
III Energy expenditure
Indirect calorimetry was performed during baseline and single-hand immersion in cool water. Continuous recordings of oxygen consumption and carbon dioxide production were made using a mask collection method (Oro-nasal reusable face mask, V7450 series, Hans Rudolph Inc., Shawnee, USA) with the Europa gas exchange monitor (GEM; Europa Scientific Ltd., Crewe, UK). Resting energy expenditure (REE) was recorded for 30 minutes before, and during, the 30 minutes hand cooling period. Average values for REE during baseline and the 30 minute hand cooling period were calculated from the last 15 minutes of each period.
IV Infrared thermography
Supraclavicular skin temperature was measured using infrared thermography (FLIR B425, thermal resolution 320x240 pixels; FLIR Systems, Danderyd, Sweden) as described previously and used as an indicator of BAT activity [21, 22, 24]. In brief, the camera was positioned so that the lens was perpendicular to the larynx and the field of view included, as a minimum, the full width of the shoulders laterally, the manubriosternal joint inferiorly and angle of the mandible superiorly. The distance from the camera required to achieve this was measured and entered alongside ambient and reflective temperatures into the thermal camera during setup as per the manufacturer’s instructions. Images were taken at 1 minute intervals during baseline and cooling. During thermographic image analysis, a region of interest (ROI) was defined as that bounded by the left sternocleidomastoid muscle, clavicle and lateral contour of the neck using ThermaCAM Researcher Pro 2.10 (FLIR systems AB, Taby, Sweden) as described previously [22, 21]. ROIs were exported into Excel (Microsoft, Redmond, WA, USA) and a custom written script in R (A Language and Environment for Statistical Computing, version 3.4.3 (R Core Team)) was used to calculate the 87.5th percentile temperature value (TSCR).
V Mean skin temperature
Mean skin temperature (TMSK) was evaluated using measurements of skin temperature obtained from wireless data loggers (iButton, model no. DS1219H-F50, resolution 0.125˚C; Maxim, Sunnyvale, CA, USA) placed at seven body sites (i.e. forehead, trunk, arm, hand, lower leg, thigh and foot) and calculated using the Hardy Du Bois formula [13]. Measurements were taken at 1 minute intervals throughout, in parallel to the acquisition of thermograms as described above.
VI Statistical analysis
All analyses were performed using GraphPad Prism for Windows Version 7 (GraphPad Software, La Jolla California USA). Data are reported as means ± SEM unless otherwise stated. All data were normally distributed as indicated by the Kolmogorov-Smirnov normality test. Comparison between baseline and 30 minute energy expenditure and skin temperature of the thigh was made using a two-tailed paired t-test. A two-way repeated measures ANOVA was used to determine whether any interaction was present between cold stimulation and ambient room temperature. Where ANOVA analysis revealed a significant F-ratio for the interaction, a post-hoc paired two-tailed t-test was employed to define the simple effect of cold stimulation at each ambient room temperature. A P value < 0.05 was considered to be statistically significant; where comparisons were made at both 18˚C and 23˚C, this threshold was adjusted (using a simple Bonferroni correction) to < 0.025.
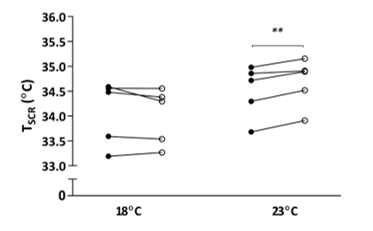
Figure 1: Effect of single-hand immersion in cool water on supraclavicular temperature at 18˚C and 23˚C ambient room temperature
A significant interaction was observed between ambient temperature and thermogenic response to hand cooling (F (1, 4) = 12.22, P = 0.025), ** P < 0.01 following 30 minutes of hand cooling (open circle) compared with baseline (circle); n = 5. TSCR – supraclavicular skin temperature
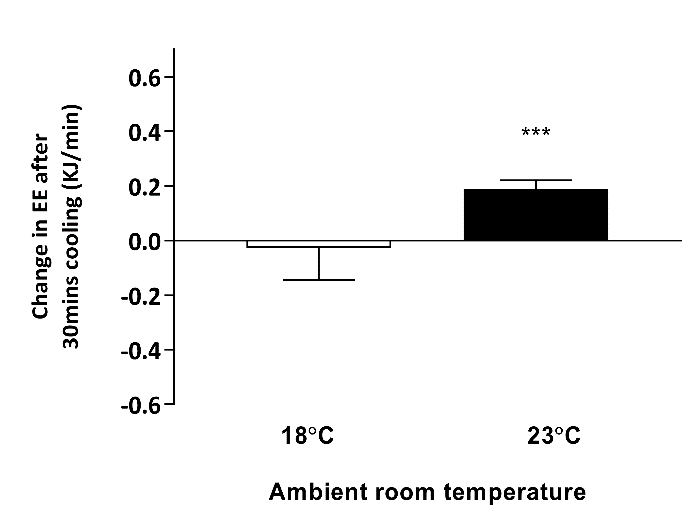
Figure 2: Change in resting energy expenditure after 30 minutes hand cooling at 18˚C and 23˚C
*** P < 0.001 when compared to baseline, n = 5. EE – energy expenditure; TSCR – supraclavicular skin temperature
Results and discussion
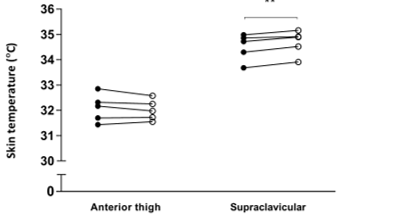
TSCR increased following single-hand immersion in cool water at an ambient room temperature of 23˚C (Figure 1). This was accompanied by a small, statistically significant increase in REE from baseline (baseline EE: 4.76 ± 0.30 kJ/min, ∆EE: 0.22 ± 0.02 kJ/min, P = 0.0008) (Figure 2). Neither change was observed in the same group of participants examined at a room temperature of 18˚C. In contrast, skin temperature measurements taken over a central location not overlying BAT (i.e. thigh) remained static at 23˚C (Figure 3), suggesting that this rise in temperature was localised to BAT, rather than secondary to a wider systemic thermogenic response.
Figure 3: Skin temperature of the anterior thigh and supraclavicular region prior to and following 30 minutes’ hand cooling at 23˚C
** P < 0.01 when compared to baseline, n = 5. Closed circles = baseline, open circles = 30 minutes cooling
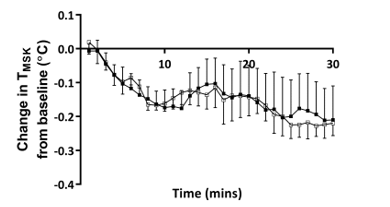
Figure 4: Time course of changes in mean skin temperature after 30 minutes hand cooling at an ambient room temperature of 18˚C and 23˚C
Closed squares = ambient temperature 23˚C, and open squares = ambient temperature 18˚C, n = 5. TMSK – mean skin temperature
Consistent with the well-described insulative response to acute cold exposure, TMSK fell from baseline throughout the duration of cooling [7]. The degree and pattern of this fall was remarkably similar at both ambient room temperatures (Figure 4), suggesting that at both 18˚C and 23˚C hand immersion in cool water acts as an effective cold stimulus of similar magnitude. Skin temperature was, as expected lower at all sites when measured at 18˚C. Moreover, the skin temperature of the non-immersed hand was well above ambient temperature during baseline at 18˚C (mean: 28.01˚C, 95% CI 23.33 to 32.70˚C) and dropped significantly (-0.61˚C, 95% CI -0.93 to -0.29˚C) following immersion of the left hand in water at 20˚C. This is consistent with the recognised vasoconstrictor response of the contralateral hand to indirect cooling, further supporting the efficacy of the cold stimulus at a cooler room temperature [19]. However, the extent to which vasoconstriction within the hand and fingers at 18˚C prior to immersion in water may have attenuated subsequent heat extraction is not certain.
The supraclavicular BAT response to acute localised cold exposure under varying ambient conditions has not been reported before. However, the outcomes of a recent study in a similar study cohort of non-obese males at 22-23˚C support our findings [1]. In that study, acute mild cold exposure prior to the onset of significant involuntary superficial muscle activity (i.e. shivering) resulted in a similar increase in energy expenditure and a similar pattern of reduction in mean skin temperature. However, supraclavicular temperature measured using a single iButton placed within the “supraclavicular zone” rather than with thermography was unchanged throughout [1]. Gashi et al (2018) also reported an isolated increase in TSCR during cooling in contrast to a fall in skin temperature at 7 other sites, in addition they identified a significant positive linear relationship between ∆TSCR and cold induced thermogenesis [12].
Ambient temperature is a determinant of FDG-detected BAT prevalence, and acute cold exposure has been shown to increase BAT activity on PET-CT [23, 25, 31]. Our findings are in line with this but are the first to show a differential effect of ambient temperature on supraclavicular heat production using thermography in response to cold stimulation, which suggests that supraclavicular BAT can be activated with minimal cold exposure. The REE and TSCR response observed at 23˚C may have been even greater had we also examined our subjects under confirmed thermoneutral conditions. One explanation for our findings is that BAT at a room temperature of 18˚C is already close to its maximal activity. However, REE at baseline was similar at 18˚C and 23˚C (4.76 ± 0.30 kJ/min and 4.88 ± 0.35 kJ/min respectively), indicating that the overall increase in REE may not be maintained long term. This may reflect a compensatory reduction in energy expenditure from another component of REE or may indicate that cold-induced BAT activation is transient.
Maximising BAT activity by increasing time spent outside of thermoneutrality presents an attractive mechanism for enhancing lipid/glucose metabolism, particularly in the context of diabetes, obesity and their metabolic sequelae. Although the changes in energy expenditure seen following cold stimulation in our study group were small (c. 3-5.5% of baseline REE), if these were sustained over a long period they could contribute significantly to energy balance. For example, assuming that 1 kg of body fat contains 37,000 kJ and a prior neutral energy balance, an increase in EE of 0.22 kJ/min for just 50% of each day would increase daily energy expenditure by 160 kJ, which, if accumulated as WAT over a single year, would equate to around 1.5 kg.
As a pilot study, our main limitation was small sample size. Nonetheless, the magnitude of the response to the mild cool stimulus at 23˚C was sufficient to reach statistical significance. How these findings may relate to a larger, more diverse population is unclear and will only be identified by further, more comprehensive studies. Although we did not observe shivering, nor did our subjects report it, EMG measurements would have enabled us to rule out subclinical muscular contraction as the cause for the increase in energy expenditure observed in our subjects. Evidence is also emerging from rodent studies for non-UCP1 dependent mechanisms of NST originating from muscle, whereby mitochondrial proton leak may be generated through UCP3 (although the role for UCP3 in humans is considered controversial), increased consumption of ATP via creatine cycling, or thermogenesis achieved via sarcolipin-mediated calcium cycling [2, 18].
Supraclavicular thermography measures infrared radiation from the skin surface, this is determined not only by the heat produced from the deeper structures such as BAT but also by an overlying “insulative layer” consisting of the skin, subcutaneous adipose tissue and also a dynamic cutaneous vasculature network. At present there are no methods to control for this, however, given that cold induced changes in supraclavicular temperature closely approximate to FDG uptake on PET-CT, and also to changes in energy expenditure we speculate that these effects are minimal [12, 14, 15,16]. As chemical-shift water-fat MRI becomes more available for the assessment of BAT, large scale studies in conjunction with perfusion assessment may enable the thermographic evaluation of BAT to be refined.
Conclusion
Our initial findings show that supraclavicular BAT can be activated under warm conditions by a very mild cold stimulus, generating heat in association with an increase in energy expenditure, and that supraclavicular BAT may be maximally stimulated at an ambient room temperature of c.18˚C. Whether sustained activation of BAT occurs at lower ambient temperatures under free-living conditions, and whether this can directly impact on overall energy balance, is unknown but presents an exciting avenue for further study.
Acknowledgments
The authors are grateful for the technical assistance of Elizabeth Simpson, Mark Pope and Sara Brown, and the administrative services of Richard Ridgwell.
Contributions
MES, HB and LJR conceived and designed the study. IAM advised on the final version of the protocol. LJR was responsible for data collection and image analysis, and MES, HB and LJR interpreted the data and IAM provided input into the final interpretation. The manuscript was drafted by LJR, MES, IAM and HB. All authors revised the manuscript, approved the final version and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Disclosures
The authors have no competing interests.
Abbreviations
BAT – brown adipose tissue
CT – computed tomography
FDG – 18F-fluorodeoxyglucose
NST – nonshivering thermogenesis
PET – positron emission tomography
REE – resting energy expenditure
TMSK – mean skin temperature
TSCR – supraclavicular skin temperature
UCP1 – uncoupling protein 1
Article Info
Article Type
Research ArticlePublication history
Received: Wed 17, Jul 2019Accepted: Mon 19, Aug 2019
Published: Fri 30, Aug 2019
Copyright
© 2023 Michael E. Symonds. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDMC.2019.01.02
Author Info
Helen Budge I A Macdonald Lindsay Jane Robinson Michael E. Symonds
Corresponding Author
Michael E. SymondsDivision of Child Health, Obstetrics and Gynaecology
Figures & Tables
A significant interaction was observed between ambient temperature and thermogenic response to hand cooling (F (1, 4) = 12.22, P = 0.025), ** P < 0.01 following 30 minutes of hand cooling (open circle) compared with baseline (circle); n = 5. TSCR – supraclavicular skin temperature
*** P < 0.001 when compared to baseline, n = 5. EE – energy expenditure; TSCR – supraclavicular skin temperature
** P < 0.01 when compared to baseline, n = 5. Closed circles = baseline, open circles = 30 minutes cooling
Closed squares = ambient temperature 23˚C, and open squares = ambient temperature 18˚C, n = 5. TMSK – mean skin temperature
References
- Acosta FM, Martinez-Tellez B, Sanchez-Delgado G, Alcantara JMA, Acosta-Manzano P et al. (2018) Physiological responses to acute cold exposure in young lean men. PLoS One 13: e0196543. [Crossref]
- Bal NC, Sahoo SK, Maurya SK, Periasamy M (2018) The Role of Sarcolipin in Muscle Non-shivering Thermogenesis. Front Physiol 9: 1217. [Crossref]
- Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA et al. (2015) Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol 29: 130-139. [Crossref]
- Bertholet AM, Kirichok Y (2018) The Mechanism FA-Dependent H(+) Transport by UCP1. Handb Exp Pharmacol 251: 143-159. [Crossref]
- Boon MR, Bakker LE, van der Linden RA, Pereira Arias-Bouda L, Smit F et al. (2014) Supraclavicular skin temperature as a measure of 18F-FDG uptake by BAT in human subjects. PLoS One 9: e98822. [Crossref]
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277-359. [Crossref]
- Castellani JW, Young AJ (2016) Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci 196: 63-74. [Crossref]
- Christensen CR, Clark PB, Morton KA (2006) Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med 31: 193-196. [Crossref]
- Cohade C, Mourtzikos KA, Wahl RL (2003) "USA-Fat": prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 44: 1267-1270. [Crossref]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509-1517. [Crossref]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R et al. (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19: 635-639. [Crossref]
- Gashi G, Madoerin P, Maushart CI, Michel R, Senn JR et al. (2019) MRI Characteristics of Supraclavicular Brown Adipose Tissue in Relation to Cold-Induced Thermogenesis in Healthy Human Adults. J Magn Reson Imaging 4. [Crossref]
- Hardy JD, Du Bois EF, Soderstrom G (1938) The technic of measuring radiation and convection: one figure. J Nutrition 15: 461-475.
- Jang C, Jalapu S, Thuzar M, Law PW, Jeavons S et al. (2014) Infrared thermography in the detection of brown adipose tissue in humans. Physiol Rep 2. [Crossref]
- Law J, Morris DE, Izzi-Engbeaya C, Salem V, Coello C et al. (2018) Thermal Imaging Is a Noninvasive Alternative to PET/CT for Measurement of Brown Adipose Tissue Activity in Humans. J Nucl Med 59: 516-522. [Crossref]
- Lee P, Bova R, Schofield L, Bryant W, Dieckmann W et al. (2016) Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab 23: 602-609. [Crossref]
- Leitner BP, Weiner LS, Desir M, Kahn PA, Selen DJ et al. (2018) Kinetics of human brown adipose tissue activation and deactivation. Int J Obes (Lond) 43: 633-637. [Crossref]
- Lin B, Coughlin S, Pilch PF (1998) Bidirectional regulation of uncoupling protein-3 and GLUT-4 mRNA in skeletal muscle by cold. Am J Physiol 275: E386-E391. [Crossref]
- Marshall JM, Stone A, Johns EJ (1990) Analysis of vascular responses evoked in the cutaneous circulation of one hand by cooling the contralateral hand. J Auton Nerv Syst 31: 57-66. [Crossref]
- Ong FJ, Ahmed BA, Oreskovich SM, Blondin DP, Haq T et al. (2018) Recent advances in the detection of brown adipose tissue in adult humans: a review. Clin Sci (Lond) 132: 1039-1054. [Crossref]
- Robinson L, Ojha S, Symonds ME, Budge H (2014) Body mass index as a determinant of brown adipose tissue function in healthy children. J Pediatr 164: 318-322. [Crossref]
- Robinson LJ, Law JM, Symonds ME, Budge H (2016) Brown adipose tissue activation as measured by infrared thermography by mild anticipatory psychological stress in lean healthy females. Exp Physiol 101: 549-557. [Crossref]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T et al. (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526-1531. [Crossref]
- Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES et al. (2016) Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab 18: 72-81. [Crossref]
- Skillen A, Currie GM, Wheat JM (2012) Thermal control of brown adipose tissue in 18F-FDG PET. J Nucl Med Technol 40: 99-103. [Crossref]
- Surasi DS, Bhambhvani P, Baldwin JA, Almodovar SE, O'Malley JP (2014) 18F-FDG PET and PET/CT patient preparation: a review of the literature. J Nucl Med Technol 42: 5-13. [Crossref]
- van Marken Lichtenbelt WD, Schrauwen P (2011) Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 301: R285-R296. [Crossref]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500-1508. [Crossref]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R et al. (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518-1525. [Crossref]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A et al. (2009) The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113-3120. [Crossref]
- Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST et al. (2009) Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging 36: 602-606. [Crossref]
