The River of no Return: Acute Aortic Dissection with Catastrophic Outcome
A B S T R A C T
The authors discuss a case of aortic dissection in a young man with hypertension. The delay in considering the diagnosis and ordering the proper investigations secondary to the unusual presentation of the case complicated by the logistics of patient’s transfer to specialized Cardiothoracic unit where surgery can be performed safely have impacted the unfortunate outcome.
Keywords
Dissecting aortic aneurysm, computer tomographic angiography dissecting flap, continuous-veno-venous hemofiltration, hypertension
Case History
29-year-old African American obese male with no self-reported past medical history presenting to the emergency department at UPMC Mercy Hospital, Pittsburgh with 3-days of bilateral flank pain and dark coca cola colored urine. Patient stated the pain is moderately severe, dull-aching and constant. Patient stated that he has not taken any medication for pain relief. He denies any history of similar symptoms, kidney stones, fevers, chills, nausea, vomiting, abdominal pain, diarrhea or constipation. He also denies any chest pain or shortness of breath at presentation. The patient did not recall any trauma or injury to the area, or any numbness or tingling of the lower extremities, or any generalized weakness. Patient does report that he's been having burning sensation on urination, change in the urine color with, small amount of urine, increased frequency and urgency of urination since back pain started.
The Emergency medical service staff reported that the patient has been hypertensive to the 200mmHg systolic in the way to the hospital, though, no interventions given to his hypertension prior to his arrival to the emergency department (ED). Review of his previous chart revealed that he had a history of hypertension documented in 2014 and was supposed to be on amlodipine and Lisinopril medications but the patient adamantly denies the use of anti-hypertensive medications or any illicit drug use.
On examination, the patient was lying comfortable in bed with mild diaphoresis. Vital signs; temperature 36.3C, BP 259/142mmHg, heart rate 95BPM, regular, and his oxygen saturation is 96% on room air. Examination of cardiovascular and respiratory systems were within normal limits, as was his abdominal examination. He has no neurological deficit and his sensation were grossly intact. His blood pressure failed to come under control despite the use of parenteral labetalol and nicardipine (233-259/116-142mmHg). His laboratory work-up reveals high blood urea nitrogen and creatinine (24/2.16mg/L), respectively, and the rest of the chemistry-panel were normal as was his complete blood picture. His urine analysis revealed the presence of 300mg of protein and moderate amount of red blood cells (RBC’s) but no casts and no evidence of infection. The electro-cardiogram (ECG) showed prolonged atrio- ventricular conduction, sinus rhythm, widespread ST and T wave inversion denoting widespread ischemia, more marked in the anterolateral wall. And prolonged QT interval (Figure 1).
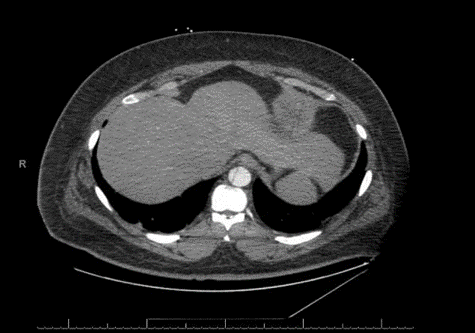
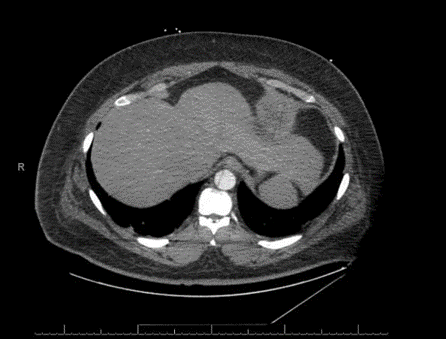
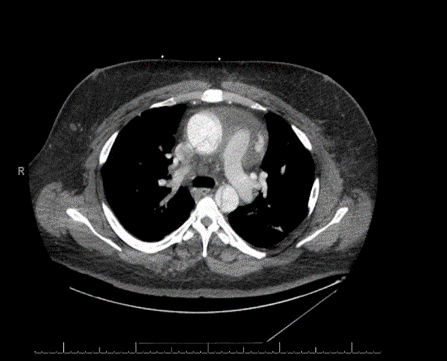
His computerized tomographic angiography (CTA) showed acute type- A aortic dissection with proximal extension involving the aortic root. The dissection flap continues into the great vessels of the arch of the aorta. The distal flap portion terminates in the right carotid artery. The left common carotid artery arises from the true lumen. Dissection flap involves the descending thoracic and abdominal aorta, celiac trunk, superior mesenteric artery (SMA), and right renal artery. The inferior mesenteric artery (IMA), and the left renal artery arise from the false lumen. There is fusiform enlargement of the ascending thoracic aorta [Figures 2, 3A, 3B, 3C]. His post-operative course was complicated by hypoxic respiratory failure requiring nitric oxide and maximal ventilator support. He developed pseudomonas pneumonia and post-operative acute renal failure requiring continuous veno-venous hemofiltration (CVVH). His blood pressure was labile and required frequent titration of multiple antihypertensive agents. On 7/19 and 7/20 his heart rhythm evolved into unstable supra-ventricular tachycardia (SVT) and atrial fibrillation with rapid ventricular rate (RVR) requiring numerous cardioversions attempts as well as amiodarone, diltiazem, and esmolol. Numerous attempts were made to contact the family at that time to inform them about the health status of the patient were without avail. On the morning of 07/21, the patient became severely hypoxic and arrested. Resuscitation were unsuccessful to revive him, and eventually he passed away.
Figure 1:
Figure 2: CTA showing the dissection flap extending to the abdominal aorta
Case Discussion
Aortic dissection (AD) is relatively uncommon, (2.6-3.5 per 100,000 person-years but often presented acutely with catastrophic illness with sudden onset “tearing or ripping pain” originating in the chest and radiating to the back [1-4]. Unlike acute coronary syndrome pain, the pain of AD is maximal at the onset and is not gradual in nature. Early and accurate diagnosis and treatment are crucial for survival. Other presentation of AD is organ hypoperfusion due to occlusion of arteries by the dissecting flap (e.g., coronary ischemia, stroke, intestinal ischemia, renal failure, limb ischemia). Aortic dissection is classified in to proximal (type-A) aortic dissection confined to the ascending aorta and the arch, which can present with acute inferior or right-sided myocardial infarction (MI) due to involvement of the right coronary artery, and distal AD (type- B) which involves the rest of the thoracic aorta and the descending abdominal aorta. Type-B AD is usually managed medically with aggressive blood pressure control. Patients with AD tend to be 60 to 80-years-old-men [5-10]. In a review of 4428 patients from the International Registry of Acute Aortic Dissection (IRAD), 66% were men with mean age of 63-years [6]. Women with AD are generally older (67-years) and have a more delayed presentation [6, 11]. Younger patients are often having history of hypertension, connective tissue disease, or anatomical abnormalities (Marfan syndrome, Ehlers-Danlos syndrome, Turners syndrome with coarctation of the aorta with bicuspid aortic valves) [12-15]. Inflammatory diseases that causes vasculitis, trauma, pregnancy and delivery, and the use of Fluoroquinolones have been implicated as risk factors and causes for AD [14-21].
Figures 3(A, B, C): Thoracic cut showing the aortic arch and the ascending aorta the dissection flap
The three major predictors of AD diagnosis are sudden, tearing chest pain; differential pulses and BP’s between the right and left arms; and abnormal aortic or mediastinal contour on chest-X ray. Transesophageal echocardiography (TEE) is fast and the most portable method for unstable patients [22-24]. The sensitivity of the TEE has been reported to be as high as 98%, and the specificity ranges from 63% - 96% [25, 26].
However, the gold-standard method for diagnosing AD is computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) [27-30]. The sensitivity of CTA ranges from 83-95% and the specificity 87-100% for the diagnosis of AD [24, 31]. The diagnosis of AD by CTA requires the identification of two distinct lumens; the intimal flap may or may not be demonstrated [31]. The MRA is an alternative to CTA but is not widely available. The sensitivity and specificity of MRA is in the range of (95-100%) [32]. D-dimer reflects activation of the extrinsic pathway of the coagulation cascade by tissue factor exposed in the aortic media by the intimal tear. D-dimer has emerged as a potential serum marker for AD [33]. However, D-dimer is non-specific and can be elevated in many conditions. Though D-dimers appears to be a useful screening tool to identify patients who do not have AD. A cutoff value of 500ng/ml; a level below this has a high negative predictive value for excluding AD [33]. In a recent systematic review, a D-dimer value of <500ng/ml have a sensitivity and specificity of (97% and 56%, respectively, and a negative predictive value of 96%) [34]. This study and others have concluded that in patients with D-dimers of <500ng/ml, aortic imaging is not going to benefit them [33-40]. A low D-dimer value with low probability of AD may be more useful for ruling out rather ruling in the diagnosis of AD.
Death from AD can be related to cardiac tamponade, acute aortic insufficiency, and acute coronary syndrome with myocardial infarction due to involvement of the right coronary artery in cases of proximal aortic dissection [41, 42]. Mortality related to AD was high, up to 30%, however, advances in cardiac surgical techniques have lowered mortality related to proximal AD to 20% [5, 6]. Significant contributing factors in the high mortality in AD is delayed time to diagnosis and transfer to cardiothoracic unit capable of performing surgery on AD. Despite all measures the mortality of AD is still unacceptably high (20-30%) even with surgery. The pathophysiology of AD can be due to iatrogenic, spontaneous (genetically mediated), or traumatic mechanisms [43]. These mechanisms can induce tear in the intima of the aorta. On the other hand, degenerative process involving the media, like cystic medial necrosis can also induce tear in the intima-media layers and causes AD. Blood passes through the tear into the aortic media separating the intima from the media and adventitia and creating a false lumen [7]. Fifty to 65% of tears originated in the ascending aorta and extends to remaining portion of thoraco-abdominal aorta [3]. Approximately, 20-30% of intimal tear originate in the vicinity of the left subclavian artery and extends into the descending thoracic and thoraco-abdominal aorta [5]. Adequate blood pressure control in the management of type-A AD is paramount. Treatment of hypertension to systolic of 110-120 mmHg using intravenous (IV) β-blockers (esmolol, labetalol) and then IV nitroprusside are indicated. Avoidance of anticoagulation and management of risk factors of AD cannot be overemphasized. Surgery is indicated for complications of dissection, end-organ damage, contained rupture, penetrating ulceration of significant size or extension despite medical therapy.
In conclusion missing the diagnosis of AD or delay in the optimal treatment and management of these unfortunate patients are often escorted with catastrophic outcome threatening the lives of these patients. Losing these patients because of our shortcomings in diagnosis and management can be devastating to the medical community and the society alike. So, listening carefully to the patient’s history, performing a detailed physical examination, considering the odd causes of patient’s symptoms along with ordering the proper investigations, and evacuating patients in a judicious manner might save lives and keep the river of life flowing.
Funding
The authors have no financial closure.
Article Info
Article Type
Case ReportPublication history
Received: Wed 09, Oct 2019Accepted: Mon 04, Nov 2019
Published: Mon 30, Dec 2019
Copyright
© 2023 Awad Magbri. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDMC.2019.01.07
Author Info
Awad Magbri Eussera El-Magbri Kaveh Ilkhanipour Kevin Leonard Michael G Abesamis
Corresponding Author
Awad MagbriUniversity of Pittsburgh Medical Center, Emergency and Toxicology Departments Pittsburgh, PA and Surgery and Vascular Center at Regency Park Toledo, OH
Figures & Tables


References
- Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ et al. (1982) Thoracic aortic aneurysms: a population-based study. Surgery 92: 1103-1108. [Crossref]
- Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L et al. (2000) Epidemiology and clinicopathology of aortic dissection. Chest 117: 1271-1278. [Crossref]
- Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM. (2004) Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 79: 176-
180. [Crossref]
- Melvinsdottir IH, Lund SH, Agnarsson BA, Sigvaldason K, Gudbjartsson T et al. (2016) The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur J Cardiothorac Surg 50: 1111-1117. [Crossref]
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ et al. (2000) The international Registry of Acute Aortic Dissection (IRAD): new insight into an old disease. JAMA 283: 897-903. [Crossref]
- Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD et al. (2018) Insight from the International Registry of Acute Aortic Dissection: a 20-year Experience of Collaborative Clinical Research. Circulation 137: 1846-1860. [Crossref]
- Larson EW, Edwards WD (1984) Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol 53: 849-855. [Crossref]
- Spittell PC, Spittell JA Jr, Joyce JW, Tajik AJ, Edwards WD et al. (1993) Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 68: 642-651. [Crossref]
- LeMaire SA, Russell L (2011) Epidemiology of thoracic aortic dissection. Nat Rev Cardiol 8: 103-113. [Crossref]
- Isselbacher EM (2014) Trends in thoracic aortic aneurysms and dissection: out of the shadows and into the light. Circulation 130: 2267- 2268. [Crossref]
- Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A et al. (2004) Gender-related differences in acute aortic dissection. Circulation 109: 3014-3021. [Crossref]
- Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE et al. (2004) Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J AM Coll Cardiol 43: 665-669. [Crossref]
- Chou AS, Ma WG, Mok SC, Ziganshin BA, Peterss S et al. (2017) Do familial aortic dissections tend to occur at the same age? Ann Thorac Surg 103: 546-550. [Crossref]
- Lin AE, Lippe B, Rosenfeld RG (1998) Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome. Pediatrics 102: e12. [Crossref]
- Carlson M, Airhart N, Lopez L, Silberbach M (2012) Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international Turner syndrome aortic dissection registry. Circulation 126: 2220-2226. [Crossref]
- Barison A, Nugara C, Barletta V, Todiere G, Montebello E et al. (2015) Asymptomatic Takayaso aortitis complicated by type-B dissection. Circulation 132: e254-e255. [Crossref]
- Khalife T, Alsac JM, Lambert M, Messas E, Duong Van Huyen JP et al. (2011) Diagnosis and surgical treatment of a Takayaso disease on an abdominal aortic dissection. Ann Vasc Surg 25: e1-e5. [Crossref]
- Smith MD, Cassidy JM, Souther S, Morris EJ, Sapin PM et al. (1995) Transesophageal echocardiography in the diagnosis of traumatic rupture of the aorta. N Engl J Med 332: 356-362. [Crossref]
- Kamel H, Roman MJ, Pitcher A, Devereux RB (2016) Pregnancy and the risk of aortic dissection or rupture: a cohort-crossover analysis. Circulation 134: 527-533. [Crossref]
- Kuperstein R, Cahan T, Yoeli-Ullman R, Ben Zekry S, Shinfeld A et al. (2017) Risk of aortic dissection in pregnant patients with the Marfan syndrome. Am J Cardiol 119: 132-137. [Crossref]
- Lee CC, Lee MG, Hsieh R, Porta L, Lee WC et al. (2018) Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol 72: 1369-1378. [Crossref]
- Blanchard DG, Kimura BJ, Dittrich HC, DeMaria AN (1994) Transesophageal echocardiography of the aorta. JAMA 272: 546-551. [Crossref]
- Cigarroa JE, Isselbacher EM, DeSanctis RW, Eagle KA (1993) Diagnostic imaging in the evaluation of suspected aortic dissection, old standards and new directions. N Engl J Med 328: 35-43. [Crossref]
- Nienaber CA, von Kodolitsch Y, Nicolas V, Siglow V, Piepho A et al. (1993) The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 328: 35-43. [Crossref]
- Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF et al. (2002) Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: International Registry of Acute Aortic Dissection (IRAD). Am J Cardiol 89: 1235-1238. [Crossref]
- Hartnell G, Costello P (1993) The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 328: 1637.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACC/AHA/AATS/ACR/ASA/SCA/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional radiology, Society of Thoracic Surgeon, and Society for Vascular Medicine. Circulation 121: 121e266.
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD et al. (2014) 2014 ESC Guidelines on the diagnosis and treatment of aortic disease: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 35: 2873-2926. [Crossref]
- Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C et al. (2010) Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 11: 645- 658. [Crossref]
- Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM et al. (2015) Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular imaging: endorsed by the Society of Cardiovascular computed tomography and society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 28: 119-182. [Crossref]
