The Association of Fgf23 and Inflammation in Heart Failure with Normal Kidney Function
A B S T R A C T
Background: Fibroblast growth factor-23 produced by osteocytes regulates calcium and phosphate homeostasis which are cornerstones for bone integrity. Recently, FGF23 was also found to be directly related with both severity and prognosis of heart failure. However, the mechanism of FGF23 regulation in heart failure, particularly in patients with preserved renal function is poorly understood.
Methods: In this retrospective single center trial we assessed the association of systemic inflammation (surrogated by CRP) and FGF23 regulation in 221 stable non-ischemic heart failure patients (age ≥ 18) with reduced ejection fraction and an estimated glomerular filtration rate of more than 60 ml/min/1.73m². Furthermore, we analyzed the prognostic ability of FGF23 and CRP in this population. Fasting ct-FGF23, highly sensitive CRP and a comprehensive panel of further biomarkers, as well as invasive hemodynamic measures from right heart catheterization, were used for univariate and multivariate regression analysis.
Results: In bivariate correlation analysis ct-FGF23 was correlated with Cardiac output (r= -0.42); NTproBNP (r=0.34) and CRP (r=0.31); for all of those p < 0.001. Multivariate linear regression analysis revealed CRP and CO as independently associated with ct-FGF23 (total model fit; r²=0.32; p <0.001). In time to event analysis ct-FGF23 was the only independent parameter predicting transplant-free survival.
Conclusion: Our data indicate an association of systemic inflammation and FGF23 in heart failure independent from renal function and supports the hypothesis that FGF23 may be directly involved in heart failure.
Keywords
Fibrosing growth factor23, C-reactive protein, heart failure , cardiomyopathy, subclinical inflammation, biomarkers
Introduction
Heart failure is seen as a multi-organ syndrome in which multiple deleterious cellular pathways are activated by humoral and mechanical mediators. For many years heart failure was seen as a consequence of hemodynamic overload, but recently growth factors and cytokines were found to play an important role in understanding heart failure [1]. Within this cytokine-model, heart failure is encompassed as a systemic inflammatory disease involving a wide range of organs [2]. C-reactive protein serves as an excellent biomarker indicating low grade inflammation and together with several other cytokines (Tumor necrosis factor alpha, Interleukin 6) is associated with heart failure even in a dose-response relationship [3, 4]. Fibroblast growth factor-23, in combination with the modulating co-receptor α-Klotho, is a phosphationic hormone produced by osteocytes and regulates calcium and phosphate homeostasis which are cornerstones for bone integrity [5]. FGF23 was extensively studied in renal dysfunction and inflammation [6-10]. Recently, FGF23 was associated with atrial fibrillation, left ventricular hypertrophy, as well as morbidity and mortality in heart failure [5, 11, 12]. However, most studies of FGF23 and heart failure are confounded by chronic kidney disease which is accompanied by disturbed mineral-, blood and Iron- homeostasis as well as inflammation – all of them interacting with FGF23 – making it difficult to explain a direct role of FGF23 on the heart [13-15].
In our study we tested the association of FGF23 and CRP in patients with non-ischemic cardiomyopathy and preserved kidney functions. We additionally studied the prognostic ability of CRP and FGF23 in this population.
Materials and Methods
We retrospectively analyzed 221 non-ischemic heart failure patients (age ≥ 18) with stable cardiomyopathy and an estimated glomerular filtration rate of more than 60 ml/min/1.73m². Study patients were retrieved from a single center heart failure registry consecutively set up from 2007 through 2013. Results from this registry were published in a former study in which however renal function was no selection criteria and burden of subclinical inflammation was not analyzed [12]. All patients were treated according prevailing heart failure guidelines. Exclusion criteria were acute heart failure, mineral and hormonal treatment for bone disturbances, obstructive coronary heart disease, chronic inflammatory disease (like inflammatory bowel disease or rheumatoid disease) and patients with an eGRF below 60 ml/min/1.73m². For time to event analysis the composite of heart transplantation and death was used. The follow up time ended December 30th (data censoring). Outcome values were retrieved by follow-up questionnaires, contacting the relatives of patients and using the local hospital patient management system or public mortality registers. The study underlies the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee of Innsbruck Medical University. All patients gave written informed consent for participation in the study.
In all patients at study entry fasting blood samples were drawn and deeply frozen (-80°C). A central laboratory measured all routine and specific parameters for this study. Highly sensitive C-reactive protein was determined by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, USA). FGF23 was measured using an FGF23 assay (Immutopics Inc., San Clemente, CA, USA). This polyclonal antibody test detects epitopes within the carboxyl-25- terminal domain (Ct-FGF23). Circulating concentrations of Ct-FGF23 are expressed as relative units per milliliter (RU/ml). Measurements of 25-hydroxyvitamin D (25(OH)D) was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Chromsystems, Munich, Germany; standardized with NIST reference material). An immunoassay was performed to measure parathyroid hormone (PTH; Modular, Roche Diagnostics GmbH, Mannheim, Germany; interassay coefficient of variation <7%). Ionic phosphate concentrations were determined by a standard colorimetric method (Modular, Roche Diagnostics GmbH, Mannheim, Germany; interassay coefficient of variation <2%). Glomerular filtration rate was estimated using the MDRD Study equation ([eGFR] [ml/min/1.73m²] = 7 175 x [serum creatinine]-1.154 x age-0.203 [x 0.742 if female]). Hemodynamic parameters were obtained by right heart catheterization and cardiac output (CO) was measured by the gold standard method using Steward Hamilton Method (thermo dilution). Left ventricular ejection (EF) was measured by echocardiography in the four-chamber view (Accuson Sequioa, Siemens).
Statistics
In this observational, retrospective study the statistical package for the social sciences (SPSS; version 17, IBM, New York, USA) was used for data analysis. Variables used for linear regression analysis are presented in (Table 1). According to skewed distributions all variables except age and body mass index were subjected to logarithmic (ln) transformation. All variables are presented as mean (± SD) and median (range) and were put in correlation except the categorical variables like New York Heart Association functional class and medication (Table 1). Pearson´s correlation was used for bivariate regression analysis and the Mann-Whitney- U Test for group comparison. Variables clinically meaningful and those significantly correlating with FGF23 were put in for stepwise multiple regression analysis. Collinearity was excluded by testing for tolerance (Tol) and variance inflation factor (VIF). Survival curves were facilitated by Kaplan Meier estimates and groups comparison with log-rank test. The prognostic value of ct-FGF23 and hs-CRP were calculated with Cox proportional hazards analysis. Proportionality was visually assessed with log-log plots.
Results
We examined 221 stable, non-ischemic heart failure patients with a median ejection fraction of 33 % (IQR 25-45%); 65 % had an EF < 40%; 85% had an EF < 50%] and an eGFR of > 60 ml/min/1.73 m² (118.32 ± 37.2) (Table 1). Bivariate correlation analysis revealed ct-FGF23 as significantly correlated with all our except age, BMI, calcium and ferritin. The highest effect size was found with CO (r= -0.42); N-terminal pro brain natrium peptide (NTproBNP, r=0.34) and CRP (r=0.31); for all of those p < 0.001 (Table 2). In the linear regression analysis ct-FGF23 was used as dependent variables and was fitted by the most meaningful explanatory variables extracted form bivariate correlation analysis. Thereby only CO and CRP remained as independent variable predicting ct-FGF23 level (Table 2). The total model fit reached: r=0.56 (r²=0.32); p < 0.001 explaining 32% of variation. In a selected group of 104 patients iron measures were available. By including iron to the model cardiac output and CRP remained to be the only independent variables. The model fit, however, increased to r²=0.45; p=<0.001; explaining 45% of variation. All models are free from relevant collinearity (Tol > 0.8 and VIF < 1.2). In a median follow-up time of 27.5 months (IQR 13.0-41.3) twenty-three events were registered (death n=16; transplantation n=7). Patients suffering an event had significantly higher FGF23 and CRP levels in comparison to those without an event [med 38.17 RU/ml (IQR; 11.16-108.28) vs. 15.83 RU/ml (IQR; 7.32-36.10); p=0.040] and [med 0.42 mg/L (IQR; 0.1-1.2) vs. 0.18 mg/L (IQR; 0.1-0.53), p=0.042] respectively.
Kaplan Meier estimates of in tertiles stratified FGF23 display an unfavorable outcome in the highest tertile only, whereas event rates regarding CRP tertiles were more linear distributed (Figure 1). In a Cox proportional hazard model with age, BMI, CO, ct-FGF23, CRP and NTproBNP as input variables FGF23 was the only independent predictor of transplant free survival (Table 3).
Table
1: Patient characteristics. Characteristics of study
population (n=221) male 70.1%.
|
mean |
SD |
median |
IQR |
||
|
Demographics |
|||||
|
age |
47.82 |
14.55 |
46 |
18 |
|
|
EF (Echo) |
% |
34.99 |
13.81 |
32.0 |
20 |
|
BMI |
kg/m² |
27.07 |
4.79 |
25.29 |
5.43 |
|
n (%) |
|||||
|
Hypertension |
83 (37.6) |
||||
|
non obstr. CAD |
28 (12.7) |
||||
|
Atrial fibrillation |
21 (9.5) |
||||
|
NYHA-FC I |
70 (31) |
||||
|
NYHA-FC II |
98 (44) |
||||
|
NYHA-FC III + IV |
51 (25) |
||||
|
Medication |
|||||
|
ACE-Inhibitors / Angiotensin Receptor
Blockers |
178 (81.7) |
||||
|
Betablockers |
165 (74.7) |
||||
|
Mineral Receptor Antagonists |
73 (33) |
||||
|
Diuretics |
116 (52.5) |
||||
|
Hemodynamics |
|||||
|
CO |
L/min |
4.20 |
1.24 |
4.1 |
1.6 |
|
PCWP |
mmHg |
17.71 |
8.62 |
16.0 |
13 |
|
mPAP |
mmHg |
27.21 |
10.66 |
25.0 |
14 |
|
mRAP |
mmHg |
10.11 |
4.82 |
9.0 |
6 |
|
Laboratory |
|||||
|
Hb |
g/dl |
14.52 |
1.58 |
14.7 |
0.17 |
|
hsCRP |
mg/L |
0.68 |
1.80 |
0.19 |
0.44 |
|
ct-FGF23 |
RU/ml |
46.72 |
100.34 |
16.83 |
30.93 |
|
Creatinin |
mg/dl |
0.96 |
0.21 |
0.91 |
0.29 |
|
eGFR |
ml/min/1.73m² |
118.32 |
37.22 |
116.70 |
43.76 |
|
Phosphate |
mg/dl |
3.34 |
0.64 |
3.41 |
0.80 |
|
Calcium |
mg/dl |
2.31 |
0.14 |
2.31 |
0.26 |
|
Parathormon |
ng/L |
41.08 |
42.16 |
32.75 |
21.47 |
|
Vit-D3 |
nmol |
50.30 |
26.67 |
47.8 |
35.9 |
|
gGT |
U/dl |
71.60 |
80.87 |
44.0 |
56.0 |
|
NTproBNP
|
ng/l |
1965 |
3602 |
921 |
2014 |
|
Ferritin (n=104) |
µmol/L |
181.66 |
184.95 |
123.0 |
67.0 |
|
Iron (n=104) |
µg/L |
17.23 |
9.30 |
16.0 |
11.0 |
ACE-I- Angiotensin converting enzyme inhibitor; BMI- body mass index;
PCWP- pulmonary capillary wedge pressure; CO- cardiac output; mRAP- mean right
atrial pressure; mPAP- mean pulmonary aterial pressure; Hb- hemoglobin; hsCRP-
high sensitie C-reactive protein; eGFR- estimated glomerular filtration rate;
NTproBNP- N-terminal pro brain natrium peptide; Vit-D3- 25-hydroxy vitamin D3;
gGT- γ-glutamyl
transferase; ct-FGF23- C- terminal fibrosing growth factor 23.
Table
2: Linear Regression Analysis - Bivariate regression analysis and
multivariate linear regression analysis (Model
II). dependent variable = ln FGF23
|
Variables |
correlations |
linear regression analysis |
||||
|
p |
Std. Regr. Coeff. |
95% Confidence Intervall |
p |
|||
|
Beta (B) |
low CI |
up CI |
||||
|
age |
0.048 |
0.05 |
-0.19 |
-0.39 |
0.01 |
ns |
|
BMI |
-0.09 |
n.s. |
||||
|
ln Hb |
-0.11 |
n.s. |
||||
|
ln CO |
0.42 |
<0.01 |
-0.24 |
-3.64 |
-0.68 |
0.004 |
|
ln mRAP |
0.29 |
<0.01 |
0.10 |
-0.36 |
1.49 |
ns |
|
ln hsCRP |
0.37 |
<0.01 |
0.21 |
0.067 |
0.39 |
0.006 |
|
ln PO4 |
0.18 |
<0.05 |
0.01 |
-0.98 |
0.96 |
ns |
|
ln PTH |
-0.26 |
<0.01 |
0.04 |
-0.33 |
0.49 |
ns |
|
ln Vit-D3 |
0.22 |
<0.01 |
-0.09 |
-0.51 |
0.16 |
ns |
|
ln gGT |
0.23 |
<0.01 |
0.07 |
-0.12 |
0.32 |
ns |
|
ln eGFR |
-0.38 |
<0.01 |
-0.52 |
-1.33 |
0.29 |
ns |
Ln- variables are subjected to logarithmic
transformation; BMI- body mass index; PCWP- pulmonary capillary wedge pressure;
CO- cardiac output; mRAP- mean right
atrial pressure; hsCRP- high sensitive C-reactive protein; PO4- phosphate; PTH-
parathyroid hormone; Vit-D3- 25 hydroxy vitamin D3; gGT - γ-glutamyl transferase; eGFR- estimated
glomerular filtration rate.
Figure 1: Kaplan Meier estimates for transplant-free survival with respect to ct-FGF23 (R/U) separated in tertiles [tertile I < 11.78; tertile II 11.79 – 31.37; tertile III > 31.37] and CRP (mg/L) [tertile I < 0.13; tertile II 0.14 – 0.377; tertile III > 0.378].
Patients with ct-FGF23 levels in the third tertile had a significant lower transplant-free survival then patient in tertile I and II (Log rank testing for both p=0.043; over all p=0.07). With respect to CRP patient in the second and third tertile had significantly lower transplant-free survival then patients in tertile I (p= 0.01 and 0.019); over all p=0.008.droxy vitamin D3; gGT - γ-glutamyl transferase; eGFR- estimated glomerular filtration rate
Table 3: Cox proportional hazard regression analysis - Predictors for transplant-free survival.
|
univariate |
multivariate |
|
||||||||||
|
variable |
Wald |
HR |
95 % CI |
p-value |
Wald |
HR |
95 % CI |
p-value |
||||
|
lower |
uper |
lower |
uper |
|||||||||
|
age |
2.55 |
1.03 |
0.99 |
1.06 |
0.11 |
0.21 |
1.01 |
0.97 |
1.04 |
0.64 |
||
|
BMI (kg/m²) |
0.01 |
1.00 |
0.91 |
1.10 |
1.02 |
1.36 |
1.067 |
0.96 |
1.19 |
0.24 |
||
|
CO (l/min) |
8.49 |
0.53 |
0.35 |
0.81 |
0.004 |
3.11 |
0.61 |
0.35 |
1.06 |
0.08 |
||
|
hsCRP |
5.15 |
1.14 |
1.02 |
1.28 |
0.02 |
0.26 |
1.06 |
0.85 |
1.33 |
0.61 |
||
|
FGF23 |
22.49 |
1.01 |
1.00 |
1.08 |
< 0.001 |
15.33 |
1.01 |
1.00 |
1.01 |
< 0.001 |
||
|
NTproBNP |
15.55 |
1.00 |
1.00 |
1.00 |
<0.001 |
3.80 |
1.00 |
1.00 |
1.00 |
0.051 |
||
BMI- body mass index; CO- cardiac output; hsCRP- highly sensitive C-reactive protein; FGF23- C- terminal fibrosing growth factor 23; NTproBNP- N-terminal pro brain natrium peptide.
Discussion
This is the first observational study investigating the relation of systemic inflammation and FGF23 in chronic, non-ischemic heart failure patients with preserved kidney functions. In bivariate correlation analysis, FGF23 correlated with many of our appointed variables but dominantly with CO. In multivariate linear regression analysis CO and CRP were the only independent predictors of FGF23. Previous studies indicate the role of FGF23 as a pro-inflammatory cytokine dependent hormone [6-9, 16]. In a large study investigating patients with chronic kidney disease, FGF23 was associated with inflammatory markers like IL-6, CRP and TNF-α [9]. Potential mechanisms of this interaction are still under discussion as FGF23 may induce inflammation and vice versa: Klotho-independent FGF23 receptors in liver and fat are capable to induce CRP and IL-6 respectively [17, 18]. A comparative genome-wide analysis of gene expression profiles showed that FGF23 responsive genes encode for pro inflammatory cytokines like lipocalin 2 and TNF-α. Furthermore, it has been demonstrated that FGF23 activates ERK-1 and phospholipase-C pathways, which are known to increase the production of IL-6 and TNF-α [19]. Importantly, this mechanism was independent of Klotho.
On the other hand, chronic subclinical inflammation effects erythropoiesis, bone and iron metabolism and via those pathways FGF23 might be indirectly induced. Various cytokines like TNF-α and NF-KB inhibit bone matrix, possibly resulting in FGF23 production by osteocytes [20]. Iron deficiency frequently observed in chronic inflammatory disease can induce FGF23 transcription, which - after an intracellular cleaving process - increases serum c-terminal FGF23 [8]. However, in contrast to previous studies, we did not find relevant associations of FGF23 with anemia and iron nor with Vit-D3 or minerals related to bone metabolism [21, 22]. From this it appears that these indirect mechanisms are of minor relevance for FGF23 regulation in heart failure with preserved renal function. Reduced cardiac output is a hallmark in heart failure. In our study cardiac output was independently associated with FGF23. Similar results were found in a former study in which authors also concluded that FGF23 contributes to heart failure progression [23]. In more recent research FGF23 was postulated to be derived from the diseased heart itself [24, 25]. Consequently a vicious cycle was proposed in which FGF23 expression could be consequence and driver of heart failure [24, 26, 27]. Our study discloses a strong association of CO and FGF23 in chronic stable heart failure patients and hence may support the hypothesis of a vicious circle. Especially in the light of the results of our Cox regression analysis in which only FGF23 was capable to predict outcome, one could conclude this hormone serves as a risk factor acting directly harmful to the heart [28, 29].
However, in the complex debate of FGF23 to be only innocent bystander or directly involved in myocardial damage, inflammation may play an important link between heart failure and FGF23 regulation apart from CDK. As mentioned above, we also observed a strong association of FGF23 with CRP. In chronic kidney disease FGF23 and inflammation seem to influence each other and independently predict mortality [30]. However, in heart failure too, there could be a positive feedback loop of FGF23 and inflammation stimulating each other. But in contrary to the observation in chronic kidney disease, in our study CRP was not an independent predictor of clinical outcome which points to a rather solely down- stream effect of FGF23 to heart failure and clinical outcome. Therefore , our study may add a little to the big puzzle of FGF23 in heart failure and may support the hypothesis of FGF23 directly involved in pathogenesis of heart failure eventually fueling a vicious circle: Low output leads to inflammation, which activates FGF23 production which perpetuates further disarrangement of cardiac structures and functions turning on the vicious cycle [31]. Our patients controversially to multiple studies dealing with FGF23 represent patients with reduced EF (85% had an EF below 50%) and are strictly inherit with apparently normal renal function. Similarly, to other studies we observed FGF23 competing with NT-pro-BNP as predictor for long term outcome [11-13, 32].
Our study has several limitations: First, despite observing only patients with eGFR > 60 ml/min/1.73 m², we cannot exclude some direct renal effects on FGF23 regulation since heart failure always interfere with renal functions. However, in our multivariate analysis phosphate, Vitamin-D3 and eGFR were not at all associated with FGF23, which makes renal influences unlikely. Second, with CRP we only “summarize” the magnitude of subclinical inflammation and therefore can only conclude on underlying cytokine activity mainly IL-6. Nevertheless, CRP as a maker of inflammation was used in multiple studies in heart failure and beyond.
Conclusion
Our data indicate an association of systemic inflammation and FGF23 in heart failure independent from renal function and support the hypothesis that FGF23 may be directly involved in heart failure.
Acknowledgements
None.
Sources of Funding
None.
Conflict of interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 08, Oct 2019Accepted: Tue 29, Oct 2019
Published: Thu 28, Nov 2019
Copyright
© 2023 Bernhard Koller. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2019.04.06
Author Info
Ulmer H Bernhard Koller Dörler J Dünser M Grander W Polzl G Zaruba MM
Corresponding Author
Bernhard KollerUniversity teaching hospital Hall in Tirol, Austria
Figures & Tables
Table
1: Patient characteristics. Characteristics of study
population (n=221) male 70.1%.
|
mean |
SD |
median |
IQR |
||
|
Demographics |
|||||
|
age |
47.82 |
14.55 |
46 |
18 |
|
|
EF (Echo) |
% |
34.99 |
13.81 |
32.0 |
20 |
|
BMI |
kg/m² |
27.07 |
4.79 |
25.29 |
5.43 |
|
n (%) |
|||||
|
Hypertension |
83 (37.6) |
||||
|
non obstr. CAD |
28 (12.7) |
||||
|
Atrial fibrillation |
21 (9.5) |
||||
|
NYHA-FC I |
70 (31) |
||||
|
NYHA-FC II |
98 (44) |
||||
|
NYHA-FC III + IV |
51 (25) |
||||
|
Medication |
|||||
|
ACE-Inhibitors / Angiotensin Receptor
Blockers |
178 (81.7) |
||||
|
Betablockers |
165 (74.7) |
||||
|
Mineral Receptor Antagonists |
73 (33) |
||||
|
Diuretics |
116 (52.5) |
||||
|
Hemodynamics |
|||||
|
CO |
L/min |
4.20 |
1.24 |
4.1 |
1.6 |
|
PCWP |
mmHg |
17.71 |
8.62 |
16.0 |
13 |
|
mPAP |
mmHg |
27.21 |
10.66 |
25.0 |
14 |
|
mRAP |
mmHg |
10.11 |
4.82 |
9.0 |
6 |
|
Laboratory |
|||||
|
Hb |
g/dl |
14.52 |
1.58 |
14.7 |
0.17 |
|
hsCRP |
mg/L |
0.68 |
1.80 |
0.19 |
0.44 |
|
ct-FGF23 |
RU/ml |
46.72 |
100.34 |
16.83 |
30.93 |
|
Creatinin |
mg/dl |
0.96 |
0.21 |
0.91 |
0.29 |
|
eGFR |
ml/min/1.73m² |
118.32 |
37.22 |
116.70 |
43.76 |
|
Phosphate |
mg/dl |
3.34 |
0.64 |
3.41 |
0.80 |
|
Calcium |
mg/dl |
2.31 |
0.14 |
2.31 |
0.26 |
|
Parathormon |
ng/L |
41.08 |
42.16 |
32.75 |
21.47 |
|
Vit-D3 |
nmol |
50.30 |
26.67 |
47.8 |
35.9 |
|
gGT |
U/dl |
71.60 |
80.87 |
44.0 |
56.0 |
|
NTproBNP
|
ng/l |
1965 |
3602 |
921 |
2014 |
|
Ferritin (n=104) |
µmol/L |
181.66 |
184.95 |
123.0 |
67.0 |
|
Iron (n=104) |
µg/L |
17.23 |
9.30 |
16.0 |
11.0 |
ACE-I- Angiotensin converting enzyme inhibitor; BMI- body mass index;
PCWP- pulmonary capillary wedge pressure; CO- cardiac output; mRAP- mean right
atrial pressure; mPAP- mean pulmonary aterial pressure; Hb- hemoglobin; hsCRP-
high sensitie C-reactive protein; eGFR- estimated glomerular filtration rate;
NTproBNP- N-terminal pro brain natrium peptide; Vit-D3- 25-hydroxy vitamin D3;
gGT- γ-glutamyl
transferase; ct-FGF23- C- terminal fibrosing growth factor 23.
Table
2: Linear Regression Analysis - Bivariate regression analysis and
multivariate linear regression analysis (Model
II). dependent variable = ln FGF23
|
Variables |
correlations |
linear regression analysis |
||||
|
p |
Std. Regr. Coeff. |
95% Confidence Intervall |
p |
|||
|
Beta (B) |
low CI |
up CI |
||||
|
age |
0.048 |
0.05 |
-0.19 |
-0.39 |
0.01 |
ns |
|
BMI |
-0.09 |
n.s. |
||||
|
ln Hb |
-0.11 |
n.s. |
||||
|
ln CO |
0.42 |
<0.01 |
-0.24 |
-3.64 |
-0.68 |
0.004 |
|
ln mRAP |
0.29 |
<0.01 |
0.10 |
-0.36 |
1.49 |
ns |
|
ln hsCRP |
0.37 |
<0.01 |
0.21 |
0.067 |
0.39 |
0.006 |
|
ln PO4 |
0.18 |
<0.05 |
0.01 |
-0.98 |
0.96 |
ns |
|
ln PTH |
-0.26 |
<0.01 |
0.04 |
-0.33 |
0.49 |
ns |
|
ln Vit-D3 |
0.22 |
<0.01 |
-0.09 |
-0.51 |
0.16 |
ns |
|
ln gGT |
0.23 |
<0.01 |
0.07 |
-0.12 |
0.32 |
ns |
|
ln eGFR |
-0.38 |
<0.01 |
-0.52 |
-1.33 |
0.29 |
ns |
Ln- variables are subjected to logarithmic
transformation; BMI- body mass index; PCWP- pulmonary capillary wedge pressure;
CO- cardiac output; mRAP- mean right
atrial pressure; hsCRP- high sensitive C-reactive protein; PO4- phosphate; PTH-
parathyroid hormone; Vit-D3- 25 hydroxy vitamin D3; gGT - γ-glutamyl transferase; eGFR- estimated
glomerular filtration rate.
Table 3: Cox proportional hazard regression analysis - Predictors for transplant-free survival.
|
univariate |
multivariate |
|
||||||||||
|
variable |
Wald |
HR |
95 % CI |
p-value |
Wald |
HR |
95 % CI |
p-value |
||||
|
lower |
uper |
lower |
uper |
|||||||||
|
age |
2.55 |
1.03 |
0.99 |
1.06 |
0.11 |
0.21 |
1.01 |
0.97 |
1.04 |
0.64 |
||
|
BMI (kg/m²) |
0.01 |
1.00 |
0.91 |
1.10 |
1.02 |
1.36 |
1.067 |
0.96 |
1.19 |
0.24 |
||
|
CO (l/min) |
8.49 |
0.53 |
0.35 |
0.81 |
0.004 |
3.11 |
0.61 |
0.35 |
1.06 |
0.08 |
||
|
hsCRP |
5.15 |
1.14 |
1.02 |
1.28 |
0.02 |
0.26 |
1.06 |
0.85 |
1.33 |
0.61 |
||
|
FGF23 |
22.49 |
1.01 |
1.00 |
1.08 |
< 0.001 |
15.33 |
1.01 |
1.00 |
1.01 |
< 0.001 |
||
|
NTproBNP |
15.55 |
1.00 |
1.00 |
1.00 |
<0.001 |
3.80 |
1.00 |
1.00 |
1.00 |
0.051 |
||
BMI- body mass index; CO- cardiac output; hsCRP- highly sensitive C-reactive protein; FGF23- C- terminal fibrosing growth factor 23; NTproBNP- N-terminal pro brain natrium peptide.
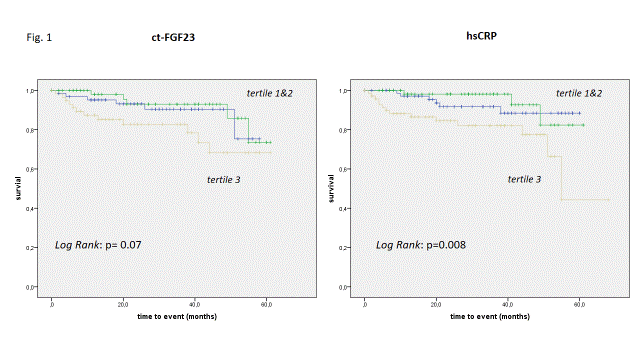
Patients with ct-FGF23 levels in the third tertile had a significant lower transplant-free survival then patient in tertile I and II (Log rank testing for both p=0.043; over all p=0.07). With respect to CRP patient in the second and third tertile had significantly lower transplant-free survival then patients in tertile I (p= 0.01 and 0.019); over all p=0.008.droxy vitamin D3; gGT - γ-glutamyl transferase; eGFR- estimated glomerular filtration rate
References
- Liu M, Chen J, Huang D, Ke J, Wu W (2014) A meta-analysis of proinflammatory cytokines in chronic heart failure. Heart Asia 6: 130-136. [Crossref]
- Dick SA, Epelman S (2016) Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res 119: 159-176. [Crossref]
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG et al. (2001) Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone Trial (VEST). Circulation 103: 2055-2059. [Crossref]
- Dupuy AM, Curinier C, Kuster N, Huet F, Leclercq F et al. (2016) Multi-Marker Strategy in Heart Failure: Combination of ST2 and CRP Predicts Poor Outcome. PLoS One 11: e0157159. [Crossref]
- Scialla JJ (2015)Epidemiologic insights on the role of fibroblast growth factor 23 in cardiovascular disease. Curr Opin Nephrol Hypertens 24: 260-267. [Crossref]
- Wolf M (2012) Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737-747. [Crossref]
- Kovesdy CP, Quarles LD (2013) Fibroblast growth factor-23: What we know, what we don’t know, and what we need to know. Nephrol Dial Transplant 28: 2228-2236. [Crossref]
- NasrAllah MM, El-Shehaby AR, Osman NA, Fayad T, Nassef A et al. (2013) The Association between Fibroblast Growth Factor-23 and Vascular Calcification Is Mitigated by Inflammation Markers. Nephron Extra 3: 106-112. [Crossref]
- Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD et al. (2012) Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7:1155-1162. [Crossref]
- Manghat P, Fraser WD, Wierzbicki AS, Fogelman I, Goldsmith DJ et al. (2010) Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int 21: 1853-1861. [Crossref]
- di Giuseppe R, Kühn T, Hirche F, Buijsse B, Dierkes J et al. (2015) Plasma fibroblast growth factor 23 and risk of cardiovascular disease: results from the EPIC-Germany case-cohort study. Eur J Epidemiol 30: 131-141. [Crossref]
- Poelzl G, Trenkler C, Kliebhan J, Wuertinger P, Seger C, et al. (2014) FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest 44: 1150-1158. [Crossref]
- Tanaka S, Fujita SI, Kizawa S, Morita H, Ishizaka N (2016) Association between FGF23, α-Klotho, and cardiac abnormalities among patients with various chronic kidney disease stages. PLoS One 11: e0156860. [Crossref]
- David V, Francis C, Babitt J (2017) Ironing out the cross talk between FGF23 and inflammation. Am J Physiol Ren Physiol 312: F1-F8. [Crossref]
- Van Breda F, Emans ME, Van Der Putten K, Braam B, Van Ittersum FJ et al. (2015) Relation between red cell distribution width and fibroblast growth factor 23 cleaving in patients with chronic kidney disease and heart failure. PLoS One 10: e0128994. [Crossref]
- Singh S, Grabner A, Yanucil C, Schramm K, Czaya B et al. (2016) Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985-996. [Crossref]
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, et al. (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393-4408. [Crossref]
- Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R et al. (2007)Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282: 26687-26695. [Crossref]
- Dai B, David V, Martin A, Huang J, Li H et al. (2012) A Comparative Transcriptome Analysis Identifying FGF23 Regulated Genes in the Kidney of a Mouse CKD Model. PLoS One 7: e44161. [Crossref]
- Rowe PS (2012)Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 22: 61-86. [Crossref]
- Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC et al. (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A 108: E1146-E1145. [Crossref]
- Braithwaite V, Jarjou LMA, Goldberg GR, Prentice A (2012)Iron status and fibroblast growth factor-23 in Gambian children. Bone 50: 1351-1356. [Crossref]
- Gutièrrez OM (2016)Fibroblast growth factor 23 and heart failure: the plot thickens. Nephrol Dial Transpl 31: 688-690. [Crossref]
- Richter M, Polyakova V, Gajawada P, Pöling J, Warnecke H, et al. Oncostatin M Induces FGF23 Expression in Cardiomyocytes. J Clin Exp Cardiol 2012: 003.
- Slavic S, Ford K, Modert M, Becirovic A, Handschuh S et al. (2017)Genetic Ablation of Fgf23 or Klotho Does not Modulate Experimental Heart Hypertrophy Induced by Pressure Overload. Sci Rep 7: 11298. [Crossref]
- Imazu M, Takahama H, Asanuma H, Funada A, Sugano Y et al. (2014)Pathophysiological impact of serum fibroblast growth factor 23 in patients with nonischemic cardiac disease and early chronic kidney disease. Am J Physiol Circ Physiol 307: H1504-H1511. [Crossref]
- Fuernau G, Pöss J, Denks D, Desch S, Heine GH et al. (2014) Fibroblast growth factor 23 in acute myocardial infarction complicated by cardiogenic shock: a biomarker substudy of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Crit Care 18: 713. [Crossref]
- Faul C (2012)Fibroblast growth factor 23 and the heart. Curr Opin Nephrol Hypertens 21: 369-375. [Crossref]
- Faul C (2018)FGF23 effects on the heart—levels, time, source, and context matter.Kidney Int 94: 7-11. [Crossref]
- Munoz Mendoza J, Isakova T, Cai X, Bayes LY, Faul C et al. (2017) Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int 91: 711-719. [Crossref]
- Stöhr R, Schuh A, Heine GH, Brandenburg V (2018) Corrigendum: FGF23 in cardiovascular disease: Innocent bystander or active mediator? Front Endocrinol (Lausanne) 9: 422. [Crossref]
- Gruson D, Lepoutre T, Ketelslegers JM, Cumps J, Ahn SA et al. (2012) C-terminal FGF23 is a strong predictor of survival in systolic heart failure. Peptides 37: 258-262. [Crossref]
