Journals
Targeting Atherosclerotic Plaque in Avicenna’s View
A B S T R A C T
Objective: Cardiovascular disease (CVD) including atherosclerosis is currently the most common cause of death in the world. Atherosclerosis can be treated by a vast variety of modalities: from lifestyle modification to invasive open surgical bypass procedures. Regarding the limitations of conventional medicine, worldwide attention to complementary and alternative medicines has increased because of their holistic approach, lower cost and better public access. In this move towards Integrative Medicine -besides other traditional schools of medicine-Persian Medicine (PM) with its long historical background should be considered as a suitable source for research.
Method: In this study we investigated major traditional literature of PM, Avicenna’s “Al-Qanun fi al-Tibb” [The Canon of medicine], to find suitable treatment modalities of atherosclerosis in comparison to conventional methods.
Result: In the quest for a concept close to atherosclerosis, “sodde” (meaning obstruction) seems to be equal to atherosclerosis and “Mofattehaat” as opener drugs with different types including “Mohallelaat” (dissolvers) and “Moghatteaat” (cutting agents) have been recommended to remove the obstructing materials. Recent studies indicate that many of the medicinal herbs which were introduced as opener drugs by Avicenna have potential pharmacological effects on managing atherosclerosis.
Conclusion: Scientific evidence confirm the efficacy of traditional herbs for elimination of atheroma. Anti-obstructive traditional medicines are similar to the conventional atherectomy in targeting atheroma by removing atherosclerotic plaque directly, but they are non-invasive, user-friendly, much cheaper and probably with less side effects.
Keywords
Atherosclerosis, Persian medicine, sodde, obstruction, Mofatteh
Traditionality
Medicine has a long background in Persian history. Avicenna (980-1037 AD) is the most eminent worldwide known Persian medicine physician and philosopher. Cardiovascular issues are widely discussed in the third volume of “al-Qānūn fī al-Tibb” (the Canon of Medicine), “Kitab al-Adviyat al-Qalbiye” (The book on drugs for cardiovascular diseases) and “Resaley-e-Ragshenasi” (treatise on Pulsology) by Ibn Sina. Obstructive diseases have been important issues for Persian scholars for hundred years ago. One of the well-known diseases in Avicenna’s books is atherosclerosis which has been described with his scientific language as obstruction of vessels. He explained the general pathophysiological principles of obstruction and its treatment.
Background
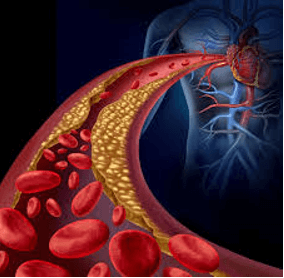
Cardiovascular diseases (CVDs) are currently the first cause of death in the world. Atherosclerosis is its most common subdivision which causes other CVDs including coronary heart, cerebrovascular and peripheral arterial diseases [1, 2]. Atherosclerosis is a dynamic and complex inflammatory disease that its first stage is fatty streak formation (Figure 1). This lesion often progresses into fibrous plaques which may be ruptured leading to thrombosis [2-5]. Atherosclerosis therapy includes a vast range of modalities beginning from lifestyle modification and medical therapy to more semi-invasive percutaneous endovascular interventions up to invasive open surgical bypass procedures or combination of these approaches. Each of these modalities has their own advantages and disadvantages [6-9].
Figure 1: Persian traditional medicine recommends “Mofattehaat” as opener drugs to remove the obstructing materials.
The most commonly used drugs include lipid lowering, antiplatelet, beta-blockers and drugs acting on the renin-angiotensin-aldosterone system (RAAS) for primary and secondary prevention and for treatment of acute coronary syndromes nitrates, fibrinolytics, antithrombins and intravenous antiplatelet drugs are consumed [6, 7, 10]. In addition, based on risk factors existing in different patients, other medications such as antihypertensive and antihyperglycemics may also be used [11]. Available drugs, besides having side effects, are mainly designed to prevent formation or progression of atheroma/clots, and according to our best knowledge, none can eliminate existing atheroma [10]. Open surgery, despite its curative nature, has obvious general risks of all surgeries and thus not very appreciated by physicians and patients unless in emergent situations [12]. Balloon angioplasty and stenting as a semi-invasive percutaneous treatment are based on pressing and displacing atheroma plaque in the arterial wall without removing it [13, 14]. Atherectomy is another semi-invasive technology using a variety of techniques such as UV light or cutting devices for atherosclerotic plaque removal [15, 16].
Regarding the limitations of conventional medicine, worldwide attention to the complementary and alternative medicines (CAM) has increased because of their holistic approach; lower cost and better public access [17, 18]. Integrating proven traditional treatments with conventional medicine is currently encouraged by The World Health Organization [19]. In this move towards Integrative Medicine -besides other traditional schools of medicine-Persian Medicine (PM) with its long historical background should be considered as a suitable and perfect source for research in complementary and alternative medicine. In this study we investigated major traditional literature of PM to find suitable treatment of atherosclerosis in comparison to the conventional methods.
Methods
In this study, the most prominent source of traditional Persian medicine (TPM), Avicenna’s “Al-Qanun fi al-Tibb” [The Canon of medicine] was searched for equivalent (Farsi and Arabic) words for obstruction and also anti-obstruction drugs. The general pathophysiological principles of obstruction, its treatment and herbal medicines were extracted and classified. A parallel search in data bases was done by using the key words of atherosclerosis and related pharmacological effects. Finally, the traditional and current mainstream paradigms of atherosclerosis treatment were compared.
Historical Background
Medicine has a long background in Iranian history. In Avesta, the oldest Iranian historical and religious text, five classes of physicians are mentioned. “Ashou bishazu” (health practitioner) who heals with hygiene, “Data bishazu” (Forensic Medicine) who works with law, “Kareto bishazu” (surgeon) who uses surgical blade for treatment, “Orvaru bishazu” (herbal physician) who treats with herbs and “Mantra bishazu” (Psychotherapist) who cure with holy words. Archaeological findings also confirm the existence of these five medical groups in ancient Persia [20]. In ancient Persia (600 BC-652 AD), medical paradigm based on the humoral theory was prevalent. Pahlavi texts from the Sassanid era (224 to 651 AD) show that scientists and physicians of that period were familiar with cardiovascular physiology and diseases such as the role of blood in feeding organs, description of pulmonary circulation, spreading infection by blood through the body and stroke [21, 22]. In the Islamic era (from seventh century AD), great Iranian scholars wrote important literatures in various fields of medicine and pharmacy. They collected and enriched various literatures from Rome, Greece, ancient Persia and India [23]. Cardiovascular diseases and their prevention and treatment is one of the major research topics of scientists of this period of medicine history [24, 25].
Although Avicenna’s view in many cases is similar to Galen’s, the most prominent Greek scholar, he also presented his own opinions, systematized the science of medicine and developed new theories in his works. Therefore, Avicenna is the most eminent worldwide known PM physician and philosopher [23, 24]. Cardiovascular issues are widely discussed in the third volume of “al-Qānūn fī al-Tibb” (the Canon of Medicine), “Kitab al-Adviyat al-Qalbiye” (The book on drugs for cardiovascular diseases) and “Resaley-e-Ragshenasi” (treatise on Pulsology) by Ibn Sina [24]. Leonardo Da Vinci (1452-1519 AD) is said to be one of the first who described atherosclerosis and after that Caleb Hillier Parry (1755-1822 AD) during his studies discovered a plaster-like substance in arteries nevertheless, obstructive diseases have been important issues for Persian scholars for hundred years ago [26]. One of the well-known diseases in Avicenna’s books (980-1037 AD), is atherosclerosis which, has been described with his scientific language as obstruction of vessels [27].
Atherosclerosis in Canon of Medicine
I Definition and Causes of Atherosclerosis (Sodde Orough)
In the quest for a concept close to atherosclerosis in Canon of Medicine, the most comparable term seems to be “sodde” meaning obstruction [27, 28]. Accumulation of materials in the vessels which prevents blood to reach the organs is called vessel obstruction “sodde orough”. Although it has been mentioned to occur in all parts of the body such as liver, spleen, kidneys, nose, nerves, brain, heart, eyes, intestines and etc., Avicenna believed that the obstruction of heart, brain and liver arteries are the most acute ones [29]. In the third volume of the Qanon, he described that arterial obstruction of heart and brain can lead to myocardial infarction and stroke, respectively [30].
Avicenna used the theory of humoral medicine to explain the causes of vascular obstruction. In Persian medicine humors includes phlegm or “balgham”, blood or “dam”, yellow bile or “safra” and black bile or “sauda”. Imbalance in the quantity and quality of these natural humors can lead to abnormal humors production and causing different diseases [31, 32]. Abnormal phlegm humor, deposition of abnormal black bile in the artery wall, yellow bile and black bile imbalance are related to cardiovascular disease risk factors and atherosclerosis [33-35]. Avicenna believes that abnormal dense "Ghaleez" or viscose “Lazej” humors accumulation is the most important cause of obstruction. The difference between dense and viscose humor in physical features is like the difference between clay and melted glue [29, 31, 36, 37]. From a holistic view, formation of fatty streak and vascular calcification which involves in the process of atherosclerotic plaque progress can be consider as viscose and dense humors respectively.
II Atherosclerosis Treatment by Opener Drugs (Mofattehaat)
To eliminate the waste materials from body they should have optimum physical properties. So, dense matter must get more fluid, and the viscous adhesive matter must be degraded into smaller pieces [31]. Certain drugs generally named openers- “Mofattehaat”- were used to remove the obstructing materials. Basically, openers with different types of functions are used according to the nature of the accumulated material to alter their rheological properties. For dense humors, dissolvers named “Mohallelaat” are used and for viscose humors, cutting agents or shredders named “Moghatteaat” are consumed [29, 38].
The "Mohallel" or dissolver is a drug that separates, dilutes and evaporates the stored humor by its hot nature [38]. This heat for removing obstructive material should be optimum, not too high nor too low. Excessive heat increases material thickness by dehydration and insufficient heat may not be effective [29]. The “Moghatte” or cutting agent / shredder is a medicine that penetrates easily into the particles. Cutting agent, due to its lower density, not only separates the viscous humor from the attached surface but also divides it to smaller fragments, therefore facilitates their removal [38]. Avicenna has introduced many herbal medicines as “Mofatteh” in the second volume of the Canon of medicine [38]. He describes the opener function of some medicines in general and for some of them he mentions the organ in which they have anti-obstructive effect. For example, the opening function of bitter almond is more than sweet almond and chicory opens the obstruction of organs and vessels [38].
Nowadays, management of atherosclerosis is carried out through modification of risk factors and processes involved in its pathogenesis such as Oxidation, inflammation, dyslipidemia, diabetes, endothelial dysfunction, platelet aggregation [39]. Table 1 presents opener drugs from Canon of medicine with examined pharmacological effects contributed in atherosclerosis treatment. It shows that many medicinal plants families can affect obstructive diseases. But Lamiaceae, Apiaceae and Asteraceae are the main referred families. Many in vivo, in vitro and clinical trial studies have been carried out on different functions of these herbs which can prove their opener effect. Most of them have anti-oxidant, Anti-dyslipidemia (by decreasing cholesterol, triglyceride, LDL and increasing HDL), anti-hyperglycemic and anti-inflammatory effects.
Table 1: Medicinal plants mentioned in the Canon of medicine as anti-obstruction treatment.
|
Scientific name |
Common name |
Traditional name |
Family |
Pharmacological effects |
Ref. |
|
Pimpinella anisum L. |
Anise |
Anison
|
Apiaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vivo), Anti-hypertensive (in vivo), Bradycardia (in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vivo), Hepatoprotective (in vitro, in vivo) |
[40-44] |
|
Tanacetum parthenium (L.) Sch.Bip. |
Feverfew |
Aqhovan |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Hepatoprotective (in vivo) |
[45-47] |
|
Lavandula stoechas L. |
Lavender |
Ostokhoddus |
Lamiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-insulin resistant (in vitro, in vitro) |
[48-50] |
|
Moringa oleifera Lam |
Moringa |
Ban |
Moringaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vivo), Anti-obesity (in vivo), Anti-coagulant and anti-platelet aggregation (in vitro), Hepatoprotective (in vivo) |
[51-58] |
|
Matricaria chamomilla Blanco |
Chamomile |
Babunaj |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemic (in vivo), Anti-obesity (in vivo), Anti-platelet aggregation (in vitro) |
[59, 60] |
|
Melissa officinalis L. |
Lemon Balm |
Badranjbuie |
Lamiaceae |
Anti-dyslipidemia (in vivo), Cardiovascular effects (human study), Anti-hypertensive (human study), Hepatoprotective (in vivo) |
[61, 62] |
|
Adiantum capillus veneris L. |
Maidenhair Fern |
Paresiavashan |
Pteridaceae |
Anti-inflammatory (in vitro, in vivo) |
[63] |
|
Ficus carica L. |
Common Fig |
Tin |
Moraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo), Anti-obesity (in vitro), Anti-hypertensive (in vitro, in vivo), Decreasing heart rate (in vitro, in vivo), Hepatoprotective (in vivo) |
[64-68] |
|
Gentiana lutea L. |
Great Yellow Gentian |
Gentiana |
Gentianaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-migratory (in vitro) |
[69] |
|
Teucrium polium L. |
Fetty Germander |
Jade |
Lamiaceae |
Anti-oxidant (in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Positive inotropic on the heart (in vivo), Anti-hypertensive (in vivo), Vasorelaxant (in vivo), Improving endothelial dysfunction (in vivo), Improving vascular inflammation (in vivo) |
[70-72] |
|
ocimum gratissimum L. |
Clove Basil |
jamsfaram |
Lamiaceae |
Anti-oxidant (in vitro), Anti-diabetic (in vivo), Anti-hypertensive (in vitro, in vivo) |
[73-75] |
|
Lawsonia inermis L. |
Henna |
Henna |
Lythraceae |
Anti-oxidant (in vitro), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Thrombolytic (in vitro), Hepatoprotective (in vivo) |
[76] |
|
Foeniculum vulgar Miller |
Fennel |
Razianaj |
Apiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti-diabetic (in vivo), Anti-thrombotic (in vivo), Anti-hypertensive (in vivo), Hepatoprotective(in vivo) |
[77, 78] |
|
Crocus sativus L. |
Saffron |
Zaferan |
Iridaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Anti-hypertensive (in vivo) |
[79-82] |
|
Nardostachys jatamansi DC |
Spikenard |
Sonbol |
Caprifoliaceae (Valerian) |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-hyperglycemic (in vitro), Anti-hypertensive (in vitro), Hepatoprotectiv (in vivo) |
[83-85] |
|
Anethum graveolens L |
Dill |
Shebet |
Apiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (human study), Anti- dyslipidemia (in vivo, human study), Anti-diabetic (in vivo, human study) |
[86-88] |
|
Aloe vera L. |
Aloe |
Sabr |
Asphodelaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo), Anti-obesity (in vivo), Prevention of vascular calcification of vascular smooth muscle cells (in vitro, in vivo), Hepatoprotective (in vivo) |
[89-93] |
|
Taraxacum officinalis Weber |
Dandelion |
Tharhashghogh |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anticoagulant and anti-platelet activity (in vitro) |
[94-96] |
|
Juniperus oxycedrus L |
Cade |
Arar |
Cupressaceae |
Anti-oxidant (in vitro, in vivo), Anti-hyperglycemic (in vivo) |
[97-99] |
|
Marrubium vulgare L. |
White Horehound |
Farasion |
Lamiaceae |
Anti-oxidant (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic, (in vivo), Hepatoprotective (in vivo) |
[100-103] |
|
Pistacia vera L. |
Pistachio |
Phostogh |
Anacardiaceae |
Anti-oxidant (in vitro, human study), Anti- inflammatory (human study), Anti- dyslipidemia (human study), Anti-diabetic (human study) |
[104, 105] |
|
Rubia tinctorum L. |
Rose Madder |
Fovvat-al-sabbaghin |
Rubiaceae |
Anti-platelet aggregation (in vitro, in vivo) |
[106] |
|
Capparis spinose L. |
Caper |
Kabar |
Capparaceae |
Anti-oxidant (, in vitro in vivo), Anti- dyslipidemia (human study), Anti-diabetic (in vivo, human study), Hepatoprotective (in vivo) |
[107-109] |
|
Apium graveolens L. |
Celery |
Karafs |
Apiaceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemia (in vivo), Vasodilator (in vivo), Decrease heart rate (in vivo), Anti-hypertensive (in vivo) |
[110-112] |
|
Cuscuta chinensis Lim. |
Chinese Dodder |
Koshoos |
Convulvulaceae |
Anti-oxidant (in vitro), Anti-diabetic (in vivo), Cardio protective (in vivo), Hepatoprotective (in vivo) |
[113] |
|
Plantago major L. |
Broadleaf Plantain |
Lesan-al-hamal |
plantaginaceae |
Anti-oxidant (in vitro, in vivo), Anti-hyperglycemic (in vivo), Anti-dyslipidemia (in vivo), Hepatoprotective (in vivo) |
[114-116] |
|
Prunus dulcis |
Almond |
Lawz |
Rosaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo, human study), Improving vascular endothelial function (in vivo), Hepatoprotective (in vivo) |
[117-122] |
|
Commiphora myrrh (Nees) Engl. |
Myrrh |
Morr |
Burseraceae |
Anti-oxidant (in vitro) |
[123] |
|
Origanum majoran L. |
Majoram |
Marzanjush |
Lamiaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-platelet activities (in vitro), Preventing proliferation of vascular smooth muscle cells (in vivo), Hepatoprotective (in vivo) |
[124-127] |
|
Trachyspermum ammi (L) Sprague ex Turrill |
Ajwain |
Nankhah |
Apiaceae |
Antioxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-platelet aggregation (in vivo), Antihypertensive (in vivo), Bradycardia (in vivo) |
[128, 129] |
|
Acorus calamus L. |
Sweet Flag |
Vajj |
Araceae |
Anti- dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo) |
[130, 131] |
|
Hypericum perforatum L. |
Perforate St John's-Wort |
Hufarigon |
Hypericaceae |
Anti-diabetic (in vivo) |
[132] |
|
Cichorium intybus L. |
Chicory |
Handeba |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemic (in vivo), Endothelium dependent vasodilation (in vivo), Anti-platelet aggregation (human study), Hepatoprotective (in vivo), Anti-hypertension (in vivo) |
[133-137] |
|
Asparagus officinalis L. |
Asparagus |
Helyoon |
Asparagaceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo, human study), Anti-hyperglycemic (in vivo, human study), Anti-hypertension (in vivo, human study) |
[138, 139] |
Discussion
Presentation of large number of medicinal plants as opener drugs demonstrates importance of treating obstructive diseases in Persian medicine. Recent studies indicate that many of the medicinal plants which were introduced as opener drugs by Avicenna have potential pharmacological effects on managing atherosclerosis. In addition, there are studies showing the direct effect of some of these herbal medicines on decreasing and removing atherosclerotic plaque.
The effect of Moringa oleifera leaf extract on reduction of atherosclerotic plaque formation on the internal carotid artery wall has been indicated in cholesterol fed rabbits [12]. Supplementation of 2% Gentiana lutea root powder in streptozotocin induced diabetic rats reduced the thickness of medial layer in aortic wall, collagen deposition and lipid and foam cell accumulation [30]. Saffron aqueous extract decreased the atherosclerotic lesion size and favorable alterations in plaque texture and enhancement of plaque stability in (ApoE-/-) mice [43].
An in vivo study by Choi et al. on high cholesterol fed rabbits indicated that Taraxacum officinale root or leaf administration decreased the atherosclerotic plaque formation in aortic walls [56]. Chicory consumption suppressed aortic cholesterol accumulation, reduced atherosclerotic lesion area and improved its stability by decreasing macrophage accumulation and increasing collagen content in ApoE-/- mice with pre-established atherosclerosis [95]. Cichorium intybus is traditionally used for blood cleansing, high blood pressure, blood purification and arteriosclerosis in Italy [140]. Yan-Sheng-Yin (YSY), a Chinese natural dietary supplement, composed of six plants which celery is its main ingredient. The ApoE-KO mice model of hyperlipidaemia was used to investigate the effects of YSY on hyperlipidaemia, atherogenesis, and obesity. The results showed reduction of visceral adipose tissues mass and adipocyte size. Also, high-dose YSY treatment decreased hepatic steatosis and atherosclerosis [141]. Amygdalin is an abundant component in almonds. Amygdalin has hypolipidemic and anti-inflammatory effect in (ApoE-/-) mice.
In atherosclerosis situation the diameter of aortic sinus and plaque area increases and the lumen area decreases which amygdalin administration improves them. D. Jiagang et al. in their study observed increase of DNA fragmentation and apoptotic cells in atherosclerotic lesions of treated animal models so they hypothesized decreasing atherosclerosis by apoptosis [83]. In addition to the effects of some herbs on removing atherosclerotic plaque, there are also invasive treatments for this purpose. Atherectomy is a potential semi-invasive method for de-bulking and removing accumulated materials from the vessels directly [16]. Laser atherectomy uses ultra-violet (UV) light to break carbon-carbon bonds. In this method, large molecules and water absorb energy and thus heated up and intracellular liquid is vaporized. Finally, the accumulated materials are destroyed. This process, also named as photo ablation, leads to dissolution and vaporization of the atherosclerotic plaque [142-144]. Different sharp cutting devices are also used to remove plaques from arterial walls in directional (DA), rotational (RA) and Orbital atherectomy (OA) methods [145-148].
As mentioned, in TPM dissolving and cutting method is used for removing accumulated material. Generally, it seems that dissolver drugs “mohallelaat” may resemble the laser atherectomy technique where in both, the accumulated material is removed by some sort of energy either from the hot nature or UV light leading to vaporization of the obstructing material. Traditional cutting agents “moghatteaat” may be comparable to directional, rotational and orbital atherectomy where they all concentrate on the fragmentation of the attached substance by using incisive means. Although, anti-obstructive traditional medicines are similar to atherectomy technique in targeting atheroma, but certainly drug therapy is preferred to invasive interventions.
Conclusion
In summary, atherosclerosis is a chronic disease which must be prevented and treated over time to prevent acute illnesses such as heart attack and stroke. Therefore, consumption of complementary therapies becomes important. CAM is noninvasive, user-friendly and has lower costs and probably less side effects in comparison to conventional therapies. By considering scientific evidence which confirms the use of traditional herbs to eliminate atheroma specifically, it is hoped that by further related researches, effective drugs could be developed from traditional Persian medicine to remove the atheroma, making better success in the treatment of atherosclerosis.
Highlight
i. In Avicenna’s viewpoint “obstruction” seems to be equivalent to atherosclerosis.
ii. Anti-obstructive drugs are known as “openers”, in Avicenna’s viewpoint.
iii. Scientific evidences confirm the efficacy of openers for elimination of atheroma.
Conflicts of Interest
None.
Article Info
Article Type
Review ArticlePublication history
Received: Wed 05, Aug 2020Accepted: Tue 01, Sep 2020
Published: Thu 10, Sep 2020
Copyright
© 2023 Sima Sadrai. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.04.14
Author Info
Maryam Yakhchali Mahdi Alizadeh Vaghasloo Mehran Mirabzadeh Ardakani Mahdi Vazirian Sima Sadrai
Corresponding Author
Sima SadraiPharmaceutical Department, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Figures & Tables
Table 1: Medicinal plants mentioned in the Canon of medicine as anti-obstruction treatment.
|
Scientific name |
Common name |
Traditional name |
Family |
Pharmacological effects |
Ref. |
|
Pimpinella anisum L. |
Anise |
Anison
|
Apiaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vivo), Anti-hypertensive (in vivo), Bradycardia (in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vivo), Hepatoprotective (in vitro, in vivo) |
[40-44] |
|
Tanacetum parthenium (L.) Sch.Bip. |
Feverfew |
Aqhovan |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Hepatoprotective (in vivo) |
[45-47] |
|
Lavandula stoechas L. |
Lavender |
Ostokhoddus |
Lamiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-insulin resistant (in vitro, in vitro) |
[48-50] |
|
Moringa oleifera Lam |
Moringa |
Ban |
Moringaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vivo), Anti-obesity (in vivo), Anti-coagulant and anti-platelet aggregation (in vitro), Hepatoprotective (in vivo) |
[51-58] |
|
Matricaria chamomilla Blanco |
Chamomile |
Babunaj |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemic (in vivo), Anti-obesity (in vivo), Anti-platelet aggregation (in vitro) |
[59, 60] |
|
Melissa officinalis L. |
Lemon Balm |
Badranjbuie |
Lamiaceae |
Anti-dyslipidemia (in vivo), Cardiovascular effects (human study), Anti-hypertensive (human study), Hepatoprotective (in vivo) |
[61, 62] |
|
Adiantum capillus veneris L. |
Maidenhair Fern |
Paresiavashan |
Pteridaceae |
Anti-inflammatory (in vitro, in vivo) |
[63] |
|
Ficus carica L. |
Common Fig |
Tin |
Moraceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo), Anti-obesity (in vitro), Anti-hypertensive (in vitro, in vivo), Decreasing heart rate (in vitro, in vivo), Hepatoprotective (in vivo) |
[64-68] |
|
Gentiana lutea L. |
Great Yellow Gentian |
Gentiana |
Gentianaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-migratory (in vitro) |
[69] |
|
Teucrium polium L. |
Fetty Germander |
Jade |
Lamiaceae |
Anti-oxidant (in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Positive inotropic on the heart (in vivo), Anti-hypertensive (in vivo), Vasorelaxant (in vivo), Improving endothelial dysfunction (in vivo), Improving vascular inflammation (in vivo) |
[70-72] |
|
ocimum gratissimum L. |
Clove Basil |
jamsfaram |
Lamiaceae |
Anti-oxidant (in vitro), Anti-diabetic (in vivo), Anti-hypertensive (in vitro, in vivo) |
[73-75] |
|
Lawsonia inermis L. |
Henna |
Henna |
Lythraceae |
Anti-oxidant (in vitro), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Thrombolytic (in vitro), Hepatoprotective (in vivo) |
[76] |
|
Foeniculum vulgar Miller |
Fennel |
Razianaj |
Apiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti-diabetic (in vivo), Anti-thrombotic (in vivo), Anti-hypertensive (in vivo), Hepatoprotective(in vivo) |
[77, 78] |
|
Crocus sativus L. |
Saffron |
Zaferan |
Iridaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vivo), Anti-hypertensive (in vivo) |
[79-82] |
|
Nardostachys jatamansi DC |
Spikenard |
Sonbol |
Caprifoliaceae (Valerian) |
Anti-oxidant (in vitro), Anti-inflammatory (in vitro), Anti-hyperglycemic (in vitro), Anti-hypertensive (in vitro), Hepatoprotectiv (in vivo) |
[83-85] |
|
Anethum graveolens L |
Dill |
Shebet |
Apiaceae |
Anti-oxidant (in vitro), Anti-inflammatory (human study), Anti- dyslipidemia (in vivo, human study), Anti-diabetic (in vivo, human study) |
[86-88] |
|
Aloe vera L. |
Aloe |
Sabr |
Asphodelaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo), Anti-obesity (in vivo), Prevention of vascular calcification of vascular smooth muscle cells (in vitro, in vivo), Hepatoprotective (in vivo) |
[89-93] |
|
Taraxacum officinalis Weber |
Dandelion |
Tharhashghogh |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anticoagulant and anti-platelet activity (in vitro) |
[94-96] |
|
Juniperus oxycedrus L |
Cade |
Arar |
Cupressaceae |
Anti-oxidant (in vitro, in vivo), Anti-hyperglycemic (in vivo) |
[97-99] |
|
Marrubium vulgare L. |
White Horehound |
Farasion |
Lamiaceae |
Anti-oxidant (in vitro, in vivo), Anti- dyslipidemia (in vivo), Anti-diabetic, (in vivo), Hepatoprotective (in vivo) |
[100-103] |
|
Pistacia vera L. |
Pistachio |
Phostogh |
Anacardiaceae |
Anti-oxidant (in vitro, human study), Anti- inflammatory (human study), Anti- dyslipidemia (human study), Anti-diabetic (human study) |
[104, 105] |
|
Rubia tinctorum L. |
Rose Madder |
Fovvat-al-sabbaghin |
Rubiaceae |
Anti-platelet aggregation (in vitro, in vivo) |
[106] |
|
Capparis spinose L. |
Caper |
Kabar |
Capparaceae |
Anti-oxidant (, in vitro in vivo), Anti- dyslipidemia (human study), Anti-diabetic (in vivo, human study), Hepatoprotective (in vivo) |
[107-109] |
|
Apium graveolens L. |
Celery |
Karafs |
Apiaceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemia (in vivo), Vasodilator (in vivo), Decrease heart rate (in vivo), Anti-hypertensive (in vivo) |
[110-112] |
|
Cuscuta chinensis Lim. |
Chinese Dodder |
Koshoos |
Convulvulaceae |
Anti-oxidant (in vitro), Anti-diabetic (in vivo), Cardio protective (in vivo), Hepatoprotective (in vivo) |
[113] |
|
Plantago major L. |
Broadleaf Plantain |
Lesan-al-hamal |
plantaginaceae |
Anti-oxidant (in vitro, in vivo), Anti-hyperglycemic (in vivo), Anti-dyslipidemia (in vivo), Hepatoprotective (in vivo) |
[114-116] |
|
Prunus dulcis |
Almond |
Lawz |
Rosaceae |
Anti-oxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo, human study), Improving vascular endothelial function (in vivo), Hepatoprotective (in vivo) |
[117-122] |
|
Commiphora myrrh (Nees) Engl. |
Myrrh |
Morr |
Burseraceae |
Anti-oxidant (in vitro) |
[123] |
|
Origanum majoran L. |
Majoram |
Marzanjush |
Lamiaceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-platelet activities (in vitro), Preventing proliferation of vascular smooth muscle cells (in vivo), Hepatoprotective (in vivo) |
[124-127] |
|
Trachyspermum ammi (L) Sprague ex Turrill |
Ajwain |
Nankhah |
Apiaceae |
Antioxidant (in vitro), Anti-inflammatory (in vivo), Anti- dyslipidemia (in vivo), Anti-platelet aggregation (in vivo), Antihypertensive (in vivo), Bradycardia (in vivo) |
[128, 129] |
|
Acorus calamus L. |
Sweet Flag |
Vajj |
Araceae |
Anti- dyslipidemia (in vivo), Anti-diabetic (in vitro, in vivo) |
[130, 131] |
|
Hypericum perforatum L. |
Perforate St John's-Wort |
Hufarigon |
Hypericaceae |
Anti-diabetic (in vivo) |
[132] |
|
Cichorium intybus L. |
Chicory |
Handeba |
Asteraceae |
Anti-oxidant (in vitro, in vivo), Anti-inflammatory (in vitro, in vivo), Anti-dyslipidemia (in vivo), Anti-hyperglycemic (in vivo), Endothelium dependent vasodilation (in vivo), Anti-platelet aggregation (human study), Hepatoprotective (in vivo), Anti-hypertension (in vivo) |
[133-137] |
|
Asparagus officinalis L. |
Asparagus |
Helyoon |
Asparagaceae |
Anti-oxidant (in vitro, in vivo), Anti-dyslipidemia (in vivo, human study), Anti-hyperglycemic (in vivo, human study), Anti-hypertension (in vivo, human study) |
[138, 139] |
References
- World health organization (2017) Cardiovascular diseases (CVDs).
- Libby P, Gaziano TA, Gaziano JM (2015) The Pathogenesis, Prevention, and Treatment of Atherosclerosis. Epidemiology of Cardiovascular Disease. In: Kasper D, Fauci A, editors. Harrison's principles of internal medicine. 19th ed, McGraw Hill education.
- Rajamani K, Fisher M (2017) An Overview of Atherosclerosis. In: Caplan LR, Biller J, editors. Primer on Cerebrovascular Diseases. 2nd ed, Academic Press 105-108.
- S C Bergheanu, M C Bodde, J W Jukema (2017) Pathophysiology and treatment of atherosclerosis current view and future perspective on lipoprotein modification treatment. Neth Heart J 25: 231-242. [Crossref]
- Herbert Plasschaert, Sylvia Heeneman, Mat J Daemen (2009) Progression in Atherosclerosis Histological Features and Pathophysiology of Atherosclerotic Lesions. Top Magn Reson Imaging 20: 227-237. [Crossref]
- Donna K Arnett, Roger S Blumenthal, Michelle A Albert, Andrew B Buroker, Zachary D Goldberger et al. (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140: e596-e646. [Crossref]
- Sidney C Smith Jr, Emelia J Benjamin, Robert O Bonow, Lynne T Braun, Mark A Creager et al. (2011) AHA/ACCF Secondary prevention and Risk Reduction Therapy for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2011 Update. A Guideline from the American Heart Association and American College of Cardiology Foundation. J Am Coll Cardiol 58: 2432-2446. [Crossref]
- Christine Parsons, Pradyumna Agasthi, Farouk Mookadam, Reza Arsanjani (2018) Reversal of Coronary Atherosclerosis: Role of LifeStyle and Medical Management. Trends Cardiovasc Med 28: 524-531. [Crossref]
- Kramer RS, Morton JR, Groom RC, Robaczewski DL (2018) Coronary Artery Bypass Grafting. In: Vasan RS, Sawyer DB. Encyclopedia of Cardiovascular Research and Medicine, Elsevier Inc 700-726.
- Khatib R, Wilson F (2018) Pharmacology of Medications Used in the Treatment of Atherosclerotic Cardiovascular Disease. Vasan RS, Sawyer DB. Encyclopedia of Cardiovascular Research and Medicine, Elsevier Inc 68-88.
- Sandra J Lewis (2009) Prevention and Treatment of Atherosclerosis: A Practitioner's Guide for 2008. Am J Med 122: S38-S50. [Crossref]
- Konstantinos Dean Boudoulas, Filippos Triposciadis, Paraschos Geleris, Harisios Boudoulas (2016) Coronary atherosclerosis: pathophysiologic basis for diagnosis and management. Prog Cardiovasc Dis 58: 676-692. [Crossref]
- Robert A Byrne, Gregg W Stone, John Ormiston, Adnan Kastrati (2017) Coronary balloon angioplasty, stents, and scaffolds. Lancet 390: 781-792. [Crossref]
- Ludman PF (2014) Percutaneous coronary intervention. Med J 42: 520-526.
- Nuri I Akkus, Abdulrahman Abdulbaki, Enrique Jimenez, Neeraj Tandon (2014) Atherectomy devices: technology update. Med Devices (Auckl) 8: 1-10. [Crossref]
- Konstantinos Katsanos, Stavros Spiliopoulos, Lazaros Reppas, Dimitris Karnabatidis (2017) Debulking Atherectomy in the Peripheral Arteries: Is There a Role and What is the Evidence? Cardiovasc Intervent Radiol 40: 964-977. [Crossref]
- Shirbeigi L, Zarei A, Naghizadeh A, Alizadeh Vaghasloo M (2017) The Concept of Temperaments in Traditional Persian Medicine. Trad Integr Med 2: 143-156.
- Hao Xu, Dazhuo Shi, Keji Chen (2012) Atherosclerosis: An Integrative East-West Medicine Perspective. Evid Based Complement Alternat Med 2012: 148413. [Crossref]
- WHO traditional medicine strategy: 2014-2023 (2013) Switzerland: World Health Organization.
- Noori A (2017) Avesta [In Persian]. Tehran: Artamis and Pejwake farzan Inc, p299.
- Arman Zargaran (2014) Ancient Persian medical views on the heart and blood in the Sassanid era (224-637 AD). Int J Cardiol 172: 307-312. [Crossref]
- Arman Zargaran, Mohammad M Zarshenas, Aliasghar Karimi, Hassan Yarmohammadi, Afshin Borhani Haghighi (2013) Management of stroke as described by Ibn Sina (Avicenna) in the Canon of Medicine. Int J Cardiol 169: 233-237. [Crossref]
- Sajjad Sadeghi, Farzaneh Ghaffari, Ghazaleh Heydarirad, Mehdi Alizadeh (2020) Galen’s place in Avicenna’s The Canon of Medicine: respect, confirmation and criticism. J Integr Med 18: 21-25. [Crossref]
- Mohammad M Zarshenas, Arman Zargaran (2015) A review on the Avicenna’s contribution to the field of cardiology. Int J Cardiol 182: 237-241. [Crossref]
- Gholamreza Kordafshari, Hoorieh Mohammadi Kenari, Mohammad Mehdi Esfahani, Mohammad Reza Shams Ardakani, Mansoor Keshavarz et al. (2015) Nutritional Aspects to Prevent Heart Diseases in Traditional Persian Medicine. Evid Based Complement Alternat Med 20: 57-64. [Crossref]
- W Slijkhuis, W Mali, Y Appelman (2009) A historical perspective towards a non-invasive treatment for patients with atherosclerosis. Neth Heart J 17: 140-144. [Crossref]
- Mb Siahpoosh, M Ebadiani, Ghr Shah Hosseini, F Nejatbakhsh (2012) Ancient theory about public health through physical activity against hyperlipidemia and ischemic heart disease. Iran J Public Health 41: 103-104. [Crossref]
- Aghili Shirazi M, Khulasah al Hikmah (2006) edited, verified and revised by Nazem E [In Persian]. Qom: Esma’ilian Publications 1: p 58.
- Avicenna H (2013) Al-Qanun fi al Tibb [The Canon of medicine] volume 1. Introduction and Scientific critical by Masoudi [In Arabic]. Tehran: Al-Mai publications.
- Sharafkandi A [Translator] (1987) The Persian translation of Qanoun fi al-tebb, Tehran: soroush Press.
- Alizadeh Vaghasloo M, Zareian MA, Soroushzadeh SMA (2016) The Concept of Nozj. Trad Integr Med 1: 133-135.
- Majid Emtiazy, Rasool Choopani, Mahmood Khodadoost, Mojgan Tansaz, Esmaiel Nazem (2013) Atheroprotector role of the spleen based on the teaching of Avicenna (Ibn Sina). Int J Cardiol 167: 26-28.
- Upur A, Jappar I (2006) The relationship between Balgham (Phlegm) and cardiovascular disease and prevention. J Med Pharma Chin Minorities 4: 77.
- Rasool Choopani, Mahmood Mosaddegh, Ashraf Al Din Gooshah Gir, Majid Emtiazy (2012) Avicenna (Ibn Sina) aspects of atherosclerosis. Int J Cardiol 156: 330. [Crossref]
- Ashraf Al din Gooshah Gir, Hasan Namdar, Elham Emaratkar, Esmaeil Nazem, Mohammad Bagher Minaii et al. (2013) Avicenna’s view on the prevention of thrombosis Int J Cardiol 166: 274-275. [Crossref]
- Ibn Nafis Qarashi A, sharh e al Qarash bar al Qanin [Qurashi's Commentary on the Canon] [In Arabic]. Tehran: Parliamentary Library of Iran Publications, 1: p 197.
- Ibn Nafis Qarashi A (2010) Al-Shamil fi al-Sana’a al-Tebbiya (part one of the third section) [In Arabic]. Tehran: Iran University of Medical Sciences, Research Institute for Islamic and Complementary Medicine.
- Avicenna H (2015) Al-Qanun fi al-Tibb [The Canon of medicine] volume 2. Introduction and Scientific critical by Masoudi [In Arabic]. Tehran: Al-Mai publication.
- Kajal A, Kishore L, Kaur N, Gollen R, Singh R (2016) Therapeutic agents for the management of atherosclerosis from herbal sources. Beni-Suef University Journal of Basic and Applied Sciences 5: 156-169.
- Victória Caroline Bottino Pontes, Daniela Pereira Rodrigues, Ariadiny Caetano, Maria Thereza Gamberini (2019) Preclinical investigation of the cardiovascular actions induced by aqueous extract of Pimpinella anisum L. seeds in rats. J Ethnopharmacol 237: 74-80. [Crossref]
- Martins N, Barros L, Santos Buelga C, Ferreira ICFR (2015) Antioxidant Potential of Two Apiaceae Plant extracts: A Comparative Study Focused on The Phenolic composition. Ind. Crops Prod.
- Ali Asadollahpoor, Mohammad Abdollahi, Roja Rahimi (2017) Pimpinella anisum L fruit: Chemical composition and effect on rat model of nonalcoholic fatty liver disease. J Res Med Sci 22: 37. [Crossref]
- Akram Jamshidzadeh, Reza Heidari, Mojtaba Razmjou, Forouzan Karimi, Mahmood Reza Moein et al. (2015) An in vivo and in vitro investigation on hepatoprotective effects of Pimpinella anisum seed essential oil and extracts against carbon tetrachloride-induced toxicity. Iran J Basic Med Sci 18: 205-211. [Crossref]
- Tepe AS, Tepe B (2015) Traditional use, biological activity potential and toxicity of Pimpinella species. Ind Crops Prod 69: 153-166.
- Yavar Mahmoodzadeh, Mohammad Mazani, Lotfollah Rezagholizadeh (2017) Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol Rep 4: 455-462. [Crossref]
- Hanganu D, Benedec D, Vlase L (2016) Polyphenolic content and antioxidant activity of Chrysanthemum parthenium extract. Farmacia 64: 498-501.
- Wu C, Chen F, Wang X (2006) Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem 96: 220-227.
- Carrasco A, Ortiz Ruiz V, Martinez Gutierrez R, Tomas V, Tudela J (2015) Lavandula stoechas essential oil from Spain: Aromatic profile determined by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Ind Crops Prod 73: 16-27.
- Celep E, Akyüz S, İnan Y, Yesilada E (2018) Assessment of potential bioavailability of major phenolic compounds in Lavandula stoechas L. ssp. Stoechas. Ind Crops Prod 118: 111-117.
- S S Kulabas, H Ipek, A R Tufekci, S Arslan, I Demirtas et al. (2018) Ameliorative potential of Lavandula stoechas in metabolic syndrome via multitarget interactions, J Ethnopharmacol 223: 88-98. [Crossref]
- Pilaipark Chumark, Panya Khunawat, Yupin Sanvarinda, Srichan Phornchirasilp, Noppawan Phumala Morales et al. (2008) The in vitro and ex vivo antioxidant properties, hypolipidemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. Leaves. J Ethnopharmacol 116: 439-446. [Crossref]
- Rajanandh MG, Satishkumar MN, Elango K, Suresh B (2012) Moringa oleifera Lam. A herbal medicine for hyperlipidemia: A preclinical report. Asian Pac J Trop Dis 2: S790-S795.
- Dolly Jaiswal, Prashant Kumar Rai, Shikha Mehta, Sanjukta Chatterji, Surekha Shukla et al. (2013) Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med 426-432. [Crossref]
- B Padayachee, H Baijnath (2019) An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S Afr J Bot 129: 304-316.
- Singh AK, Rana HK, Tshabalala T (2019) Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. S Afr J Botany.
- B Y Aju, R Rajalakshmi, S Mini (2019) Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats. Heliyon 5: e02935. [Crossref]
- Vasanth K, Minakshi GC, Ilango K, Kumar RM, Agrawal A et al. (2015) Moringa oleifera attenuates the release of pro-inflammatory cytokines in lipopolysaccharide stimulated human monocytic cell line. Ind Crops Prod 77: 44-50.
- Metwally FM, Rashad HM, Ahmed HH, Mahmoud AA, ER abdol Raouf et al. (2017) Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac J Trop Biomed 7: 214-221.
- Michał Bijak, Joanna Saluk, Marta Tsirigotis Maniecka, Halina Komorowska, Barbara Wachowicz et al. (2013) The influence of conjugates isolated from Matricaria chamomilla L. on platelets activity and cytotoxicity. Int J Biol Macromol 61: 218-229. [Crossref]
- Mohamed Amine Jabri, Mohsen Sakly, Lamjed Marzouki, Hichem Sebai (2017) Chamomile (Matricaria recutita L.) decoction extract inhibits in vitro intestinal glucose absorption and attenuates high fat diet-induced lipotoxicity and oxidative stress. Biomed pharmacother 87: 153-159. [Crossref]
- Zare Javid A, Haybar H, Dehghan P (2018) The effects of melissa officinalis on echocardiography, exercise test, serum biomarkers, and blood pressure in patients with chronic stable angina. J Herb Med.
- S Bolkent, R Yanardag, Omur Karabulut Bulan, B Yesilyaprak (2005) Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: A morphological and biochemical study. J Ethnopharmacol 99: 391-398. [Crossref]
- Qianying Yuan, Xuenong Zhang, Ziwei Liu, Shanshan Song, Pinpin Xue et al. (2013) Ethanol extract of Adiantum capillus-veneri L. suppresses the production of inflammatory mediators by inhibiting NF-кB activation. J Ethnopharmacol 147: 603-611. [Crossref]
- Olfa Belguith Hadriche, Sonda Ammar, Maria Del Mar Contreras, Mouna Turki, Antonio Segura Carretero et al. (2016) Antihyperlipidemic and Antioxidant Activities of Edible Tunisian Ficus carica L. Fruits in High Fat Diet-Induced Hyperlipidemic Rats. Plant Foods Hum Nutr 71:183-189. [Crossref]
- Santiagu Stephen Irudayaraj, Sunil Christudas, Stalin Antony, Veeramuthu Duraipandiyan, Al Dhabi Naif Abdullah et al. (2017) Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharm Biol 55: 1074-1081. [Crossref]
- Abdullah Turan, Ismail Celik (2016) Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity. Inter J Biol Macromol 91: 554-559. [Crossref]
- Ramgopal Mopuri, Muniswamy Ganjayi, Balaji Meriga, Neil Anthony Koorbanally, Md Shahidul Islam (2018) The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J Food Drug Anal 26: 201-210. [Crossref]
- Alamgeer, Shifa Iman, Hira Asif, Muhammad Saleem (2017) Evaluation of antihypertensive potential of Ficus carica fruit. Pharm Biol 55: 1047-1053. [Crossref]
- R Kesavan, S Chandel, S Upadhyay, R Bendre, R Ganugula et al. (2016) Gentiana lutea exerts anti-atherosclerotic effects by preventing endothelial inflammation and smooth muscle cell migration. Nutr Metab Cardiovasc Dis 26: 293-301. [Crossref]
- Parvaneh Sadat Tabatabaie, Razieh Yazdanparast (2017) Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed Pharmacother 93: 1033-1039. [Crossref]
- Seed Niazmand, Maryam Esparham, Tahereh Hassannia, Mohammad Derakhshan (2011) Cardiovascular effects of Teucrium polium L. extract in rabbit. Phcog Mag 7: 260-264. [Crossref]
- Sakineh Khodadadi, Narges Amel Zabihi, Saeed Niazmand, Abbasali Abbasnezhad, Maryam Mahmoudabady et al. (2018) Teucrium polium improves endothelial dysfunction by regulating eNOS and VCAM-1 genes expression and vasoreactivity in diabetic rat aorta. Biomed Pharmacother 103: 1526-1530. [Crossref]
- Shaw HM, Wu JL, Wang MS (2017) Antihypertensive effects of Ocimum gratissimum extract: Angiotensin-converting enzyme inhibitor in vitro and in vivo investigation. J Funct Foods 35: 68-73.
- Antora RA, Salleh RM (2017) Antihyperglycemic effects of Ocimum plants: A short review. Asian Pac J Trop Biomed 7: 755-759.
- Santanu Kar Mahapatra, Somenath Roy (2014) Phytopharmacological approach of free radical scavenging and antioxidative potential of eugenol and Ocimum gratissimum Linn. Asian Pac J Trop Med 7: S391-S397. [Crossref]
- Ruchi Badoni Semwal, Deepak Kumar Semwal, Sandra Combrinck, Catherine Cartwright Jones, Alvaro Viljoen (2014) Lawsonia inermis L. (henna): Ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol 155: 80-103. [Crossref]
- Rather MA, Dar BA, Sofi SN, Bhat BA, Qurishi MA (2016) Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab J Chem 9: S1574-S1583.
- Ahmed AF, Shi M, Liu C, Kang W (2019) Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Science and Human Wellness 8: 67-72.
- Olga Mykhailenko, Volodymyr Kovalyov, Olga Goryacha, Liudas Ivanauskas, Victoriya Georgiyants (2019) Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 162: 56-89. [Crossref]
- Samad Ghaffari, Neda Roshanravan (2019) Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed Pharmacother 109: 21-27. [Crossref]
- Saeed Samarghandian, Mohsen Azimi Nezhad, Tahereh Farkhondeh (2017) Immunomodulatory and antioxidant effects of saffron aqueous extract (Crocus sativus L.) on streptozotocin-induced diabetes in rats. Indian Heart J 69: 151-159. [Crossref]
- Ei Christodoulou, N P E Kadoglou, M Stasinopoulou, O A Konstandi, C Kenoutis et al. (2018) Crocus sativus L. aqueous extract reduces atherogenesis, increases atherosclerotic plaque stability and improves glucose control in diabetic atherosclerotic animals. Atherosclerosis 268: 207-214. [Crossref]
- Surendra Kumar Sharma, Ajay Pal Singh (2012) In Vitro Antioxidant and Free Radical Scavenging Activity of Nardostachys jatamansi DC. J Acupunct Meridian Stud 5: 112-118. [Crossref]
- Biswajit Bose, Debabrata Tripathy, Anupam Chatterjee, Pramod Tandon, Suman Kumaria (2018) Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of Nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine 55:58-69. [Crossref]
- S Ali, K A Ansari, M A Jafry, H Kabeer, G Diwakar (2000) Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats. J Ethnopharmacol 71: 359-363. [Crossref]
- Ebrahim Abbasi Oshaghi, Iraj Khodadadi, Heidar Tavilani, Mohammad Taghi Goodarzi (2016) Aqueous Extract of Anethum Graveolens L. has Potential Antioxidant and Antiglycation Effects. Iran J Med Sci 41: 328-333. [Crossref]
- Payahoo L, Ostadrahimi A, Mobasseri M (2014) Anethum graveolens L. supplementation has Anti-inflammatory effect in type 2 diabetic patients. Indian J Tradit Know 13: 461-465.
- Mohammad Taghi Goodarzi, Iraj Khodadadi, Heidar Tavilani, Ebrahim Abbasi Oshaghi (2016) The Role of Anethum graveolens L. (Dill) in the Management of Diabetes. J Trop Med 2016: 1098916. [Crossref]
- Mahesh Sapkota, Saroj Kumar Shrestha, Ming Yang, Young Ran Park, Yunjo Soh (2019) Aloe-emodin inhibits osteogenic differentiation and calcification of mouse vascular smooth muscle cells. Eur J Pharmacol 865: 172772. [Crossref]
- Ramesh Kumar, Amit Kumar Singh, Ashutosh Gupta, Anupam Bishayee, Abhay K Pandey (2019) Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine 60: 152996. [Crossref]
- Maharjan H Radha, Nampoothiri P Laxmipriya (2015) Evaluation of biological properties and clinical effectiveness of Aloe vera: Asystematic review. J Tradit Complement Med 5: 21-26. [Crossref]
- Walid R, Hafida M, Abdelhamid EHI, Reda B, Rachid A, Mohamed B (2018) Beneficial effects of Aloe vera gel on lipid profile, lipase activities and oxidant/antioxidant status in obese rats. J Funct Foods 48: 525-532.
- Abo Youssef AMH, Messiha BAS (2013) Beneficial effects of Aloe vera in treatment of diabetes: Comparative in vivo and in vitro studies. Bulletin of Faculty of Pharmacy, Cairo University 51: 7-11.
- Dariusz Jędrejek, Bogdan Kontek, Bernadetta Lis, Anna Stochmal, Beata Olas (2017) Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem Biol Interact 262: 29-37. [Crossref]
- Ung Kyu Choi, Ok Hwan Lee, Joo Hyuk Yim, Chang Won Cho, Young Kyung Rhee et al. (2010) Hypolipidemic and Antioxidant Effects of Dandelion (Taraxacum officinale) Root and Leaf on Cholesterol-Fed Rabbits. Int J Mol Sci 11: 67-78. [Crossref]
- Bernadetta Lis, Dariusz Jędrejek, Anna Stochmal, Beata Olas (2018) Assessment of effects of phenolic fractions from leaves and petals of dandelion in selected components of hemostasis. Food Res Int 107: 605-612. [Crossref]
- Chaouche TM, Haddouchi F, Atik Beraka F (2014) Antioxidant, haemolytic activities and HPLC-DAD-ESI-MSn characterization of phenolic compounds from root bark of Juniperus oxycedrus subsp. oxycedrus. Ind Crops Prod.
- Nilüfer Orhan, Aysel Berkkan, Didem Deliorman Orhan, Mustafa Aslan, Fatma Ergun (2011) Effects of Juniperus oxycedrus ssp. Oxycedrus on tissue lipid peroxidation, trace elements (Cu, Zn, Fe) and blood glucose levels in experimental diabetes. J Ethnopharmacol 133: 759-764. [Crossref]
- Nilüfer Orhan, Mustafa Aslan, Betül Demirci, Fatma Ergun (2012) A bioactivity guided study on the anti-diabetice activity of Juniperus oxycedrus ssp. Oxycedrus L. leaves. J Ethnopharmacol 140: 409-415. [Crossref]
- Amessis Ochemoukh N, Abu Reidah IM, Quirantes Pine R, Madani K, Segura Carretero A (2014) Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind Crops Prod 61: 120-129.
- Abeer Y Ibrahim, Saber F Hendawy, Ahmed A A Elsayed, Elsayed A Omer (2016) Evaluation of hypolipidemic Marrubium vulgare effect in Triton WR-1339-induced hyperlipidemia in mice. Asian Pac J Trop Med 9: 453-459. [Crossref]
- Elbery AA, Harraz FM, Ghareib SA, Gabr SA, Nagy AA et al. (2015) Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslidemia in streptozotocin-induced diabetic rats. Int J Diabetes Mellit 3: 37-44.
- Akther N, Shawl AS, Sultana S, Chandan BK, Akther M (2013) Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J Pharm Res 565-670.
- Davide Barreca, Giuseppina Laganà, Ugo Leuzzi, Antonella Smeriglio, Domenico Trombetta et al. (2016) Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L. variety Bronte) hulls. Food Chem 493-502. [Crossref]
- Seema Gulati, Anoop Misra, Ravindra Mohan Pandey, Surya Prakash Bhatt, Shelza Saluja (2014) Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: A 24-wk, randomized control trial. Nutrition 30: 192-197. [Crossref]
- Fatima Zahra Marhoume, Mehdi Ait Laaradia, Younes Zaid, Jawad Laadraoui, Sara Oufquir et al. (2019) Anti-aggregant effect of butanolic extract of Rubia tinctorum L on platelets In vitro and ex vivo. J Ethnopharmacol 241: 111971. [Crossref]
- Nizar Tlili, Walid Elfalleh, Ezzeddine Saadaoui, Abdelhamid Khaldi, Saida Triki et al. (2011) The caper (Capparis L.): Ethnopharmacology, phytochemical and pharmacological properties. Fitoteralia 82: 93-101. [Crossref]
- Gull T, Anwar F, Sultana B, Alcayde MAC, Nouman W (2015) Capparis species: A potential source of bioactives and high-value components: A review. Ind Crops Prod 67: 81-96.
- Hamideh Vahid, Hassan Rakhshandeh, Ahmad Ghorbani (2017) Antidiabetic properties of Capparis spinose L. and its components. Biomed Pharmacother 92: 293-302. [Crossref]
- Wesam Kooti, Nahid Daraei (2017) A review of the antioxidant Activity of Celery (Apium graveolens L). Evid.-Based Complementary Altern Med 22: 1029-1034. [Crossref]
- Tashakori Sabzevar F, Razavi BM, Imenshahidi M (2016) Evaluation of mechanism for antihypertensive and vasorelaxant effects of hexanic and hydroalcoholic extracts of celery seed in normotensive and hypertensive rats. Rev Bra Farmacogn 26: 619-626.
- Faezeh Tashakori Sabzevar, Masoud Ramezani, Hossein Hosseinzadeh, Seyyed Mohammad Reza Parizadeh, Ahmad Reza Movassaghi et al. (2016) Protective and hypoglycemic effects of celery seed on streptozotocin-induced diabetic rats: experimental and histopathological evaluation. Acta Diabetol 53: 609-619. [Crossref]
- Sineeporn Donnapee, Jin Li, Xi Yang, Ai hua Ge, Paul Owusu Donkor et al. (2014) Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J Ethnopharmacol 157: 292-308. [Crossref]
- Piyaviriyakul KS, Vallisuta PSO (2014) HPTLC simultaneous quantification of triterpene acids for quality control of Plantago major L. and evaluation of their cytotoxic and antioxidant activities. Ind Crops Prod 60: 239-246.
- Xiaolong Ji, Chunyan Hou, Xudan Guo (2019) Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int J Biol Macromol 135: 637-646. [Crossref]
- Hussan F, Basah RHO, Yusof MRM, Kamaruddin NA, Othman F (2015) Plantago major treatment enhanced innate antioxidant activity in experimental acetaminophen toxicity. Asian Pac J Trop Biomed 5: 728-732.
- Edward Bitok, Joan Sabaté (2018) Nuts and Cardiovascular Disease. Prog Cardiovasc Dis 61: 33-37. [Crossref]
- Kleopatra Alexiadou, Nicholas Katsilambros (2011) Nuts: anti-atherogenic food? Eur J Intern Med 22: 141-146. [Crossref]
- Mohammad Javad Zibaeenezhad, Parham Ostovan, Seyed Hamdollah Mosavat, Mahmood Zamirian, Armin Attar (2018) Almond oil for patients with hyperlipidemia: A randomized open label controlled clinical trial. Complement Ther Med 2018. [Crossref]
- Ravindra Bhardwaj, Harvinder Dod, Manjinder S Sandhu, Rohil Bedi, Sachin Dod et al. (2018) Acute effects of diets rich in almond and walnuts on endothelial function. Indian Heart J 70: 497-501. [Crossref]
- Jia XY, Zhang QA, Zhang ZQ (2011) Hepatoprotective effects of almond oil against carbon tetrachloride induced liver injury in rats. Food Chem 125: 673-678.
- Deng Jiagang, Chunyang Li, Hailian Wang, Erwei Hao, Zhengcai Du et al. (2011) Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochem Biophys Res Commun 411: 523-529. [Crossref]
- Tao Shen, Guo Hui Li, Xiao Ning Wang, Hong Xiang Lou (2012) The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 142: 319-330. [Crossref]
- Wahby MM, Yacout G, Kandeel K, Awad D (2015) LPS-induced oxidative inflammation and hyperlipidemia in male rats: the protective role of Origanum majorana extract. Beni-Suef University Journal of Basic and Applied Sciences 4: 291-298.
- Hamdy Roby MH, Sarhan MA, Selim KAH, Khalel KI (2013) Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.) and marjoram (Origanum majorana L.) extracts. Ind Crops Prod 43: 827-831.
- Razieh Yazdanparast, Leila Shahriyary (2008) Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vasc Pharmacol 48: 32-37. [Crossref]
- Yu YM, Tzeng YW (2009) Carnosic acid & ursolic acid (Origanum Majorana L.) prevent atherosclerosis caused by obesity: Suppression of leptin-induced proliferation in vascular smooth muscle cells. Atherosclerosis 10: supplement.
- Asif HM, Sultana S, Akhtar N (2014) A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of Trachyspermum ammi Linn. Asian Pac J Trop Biomed 4: S545-S553.
- Ranjbaran A, Kavoosi G, Mojallal-Tabatabaei Z, Ardestani SK (2019) The antioxidant activity of Trachyspermum ammi essential oil and thymol in murine macrophages. Biocatalysis and Agricultural Biotechnology 20: 101220.
- Yun Xi Liu, Mei Mei Si, Wei Lu, Li Xia Zhang, Chang Xin Zhou et al. (2015) Effects and molecular mechanisms of the antidiabetic fraction of Acorus calamus L. on GLP-1 expression and secretion in vivo and in vitro. J Ethnopharmacol 166: 168-175. [Crossref]
- Reshma S Parab, Sushma A Mengi (2002) Hypolipidemic activity of Acorus calamus L. in rats. Fitoterapia 73: 451-455. [Crossref]
- S Arokiyaraj, R Balamurugan, P Augustian (2011) Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 1: 386-390. [Crossref]
- Al Snafi AE (2016) Medical importance of Cichorium intybus - A review. IOSR J Pharm 6: 41-56.
- Weiqun Lin, Chaoqun Liu, Hai Yang, Wenting Wang, Wenhua Ling et al. (2015) Chicory, a typical vegetable in Mediterranean diet, exerts a therapeutic role in established atherosclerosis in apolipoprotein E-deficient mice. Mol Nutr Food Res 59: 1803-13. [Crossref]
- Liu C, Wang W, Lin W, Ling W, Wang D (2016) Established atherosclerosis might be a perquisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol Nutr Food Res.
- Abdul Malik, Malik Hassan Mehmood, Hajra Channa, Muhammad Shoaib Akhtar, Anwarul Hassan Gilani (2017) Pharmacological basis for the medicinal use of polyherbal formulation and its ingredients in cardiovascular disorders using rodents. BMC Complementary and Alternative Medicine 17: 142. [Crossref]
- Edit Schumacher, Eva Vigh, Valéria Molnár, Péter Kenyeres, Gergely Fehér et al. (2011) Thrombosis Preventive Potential of Chicory Coffee Consumption: A Clinical Study. Phytother Res 25: 744-748. [Crossref]
- Guo Q, Wang N, Liu H, Li Z, Lu L et al. (2019) The bioactive compounds and biological functions of Asparagus officinalis L. - A review. J Funct Foods.
- Mie Nishimura, Tatsuya Ohkawara, Hiroyo Kagami Katsuyama, Hiroji Sato, Jun Nishihira (2013) Improvement of Blood Pressure, Glucose Metabolism, and Lipid Profile by the Intake of Powdered Asparagus (Lú Sǔn) Bottom-stems and Cladophylls. J Tradit Complement Med 3: 250-255. [Crossref]
- Renée A Street, Jasmeen Sidana, Gerhard Prinsloo (2013) Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid Based Complementary Altern Med 2013: 579319. [Crossref]
- Wu CH, Pan CH, Lee CK (2016) Anti-obesity and anti-hyperlipidaemic effects of Yan-Sheng-Yin in animals and humans. J Funct Foods 24: 173-182.
- John Rawlins, Jehangir N Din, Suneel Talwar, Peter O'Kane (2016) Coronary Intervention with the Excimer Laser: Review of the Technology and Outcome Data. Interventional Cardiology Review 11: 27-32. [Crossref]
- Jérémie Jayet, Raphaël Coscas, Frédéric Heim, Olivier Goeau Brissonniere, Isabelle Javerliat et al. (2018) Laser Uses In Non-Coronary Arterial Disease. Ann Vasc Surg 57: 229-237. [Crossref]
- Keisuke Nakabayashi, Daisuke Sunaga, Nobuhito Kaneko, Akihiro Matsui, Kazuhiko Tanaka et al. (2018) Simple percutaneous coronary interventions using the modification of complex coronary lesion with excimer laser. Cardiovasc Revasc Med 20: 293-302. [Crossref]
- Tariq M Bhat, Maxwell E Afari, Lawrence A Garcia (2017) Atherectomy in Peripheral Artery Disease: A Review. J Invasive Cardiol 29: 135-144. [Crossref]
- Trung Tran, Michael Brown, John Lasala (2008) An Evidence-Based Approach to the Use of Rotational and Directional Coronary Atherectomy in the Era of Drug-Eluting Stents: When Does It Make Sense? Catheter Cardiovasc Interv 72: 650-662. [Crossref]
- Jay S Shavadia, Minh N Vo, Kevin R Bainey (2018) Challenges With Severe Coronary Artery Calcification in Percutaneous Coronary Intervention: A Narrative Review of Therapeutic Options. Can J Cardiol 34: 1564-1572. [Crossref]
- Evan Shlofmitz, Brad J Martinsen, Michael Lee, Sunil V Rao, Philippe Généreux et al. (2017) Orbital atherectomy for the treatment of severely calcified coronary lesions: evidence, technique, and best practices. Expert Rev Med Devices 14: 867-879. [Crossref]
