Successful Endovascular Treatment of Trigeminal Neuralgia Caused by a CarotidCavernous Fistula: Case Report
A B S T R A C T
Dural arteriovenous fistulas (DAVFs) of the cavernous sinus are arteriovenous connections located in the dura mater leaflets of this region. The usual presentation of a DAVF is predominantly ocular, with symptoms such as diplopia, conjunctival injection, involvement of cranial nerves III/IV/VI, exophthalmos, and chemosis. Trigeminal neuralgia caused by a cavernous DAVF is extremely rare. To the best our knowledge, this is only the fourth report in the world literature. We describe the case of a patient treated by embolization in whom the only presenting symptom of DAVF was trigeminal neuralgia. After endovascular treatment, the patient became asymptomatic.
Keywords
Brain angiogram, carotid cavernous fistula, embolization, trigeminal neuralgia
Introduction
Dural arteriovenous fistulas (DAVFs) of the cavernous sinus are arteriovenous connections located in the dura mater leaflets of this region [1]. The usual presentation of a DAVF is predominantly ocular, with symptoms such as diplopia, conjunctival hyperemia, involvement of cranial nerves III/IV/VI, exophthalmos, and chemosis [2-5]. The patient may rarely present with facial pain as a concomitant symptom [6-9]. This pain is usually due to irradiation from the ocular region, not to involvement of the trigeminal nerve. Trigeminal neuralgia caused by a cavernous DAVF is a very rare entity and has only been reported three times in the published literature. We describe the case of a patient who presented with trigeminal neuralgia as the main symptom of a cavernous DAVF, without any concomitant ocular symptoms.
Case Presentation
A 46-year-old female smoker sought care with a chief complaint of multiple daily episodes of shock-like right temporal headache and facial pain in a V1/V2 dermatome distribution, of more than 2 years’ duration. A clinical diagnosis of trigeminal neuralgia was established. Conservative treatment had proved ineffective. Physical and neurological examination were within normal limits. The patient undergone balloon compression of the trigeminal ganglion after 6 months of clinical treatment with partial relief of pain lasting approximately 3 months and refused to undergo further procedures. Investigation with magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography of the cerebral and cervical vessels; both were normal, with no evidence of neurovascular compression in the trigeminal territory, before and after ballon treatment. Her symptoms continued to deteriorate, with headache and severe facial pain. The decision was made to perform angiography for diagnostic clarification. The diagnostic hypotheses were sinus thrombosis or a dural fistula not demonstrated by MRI and MR angiography.
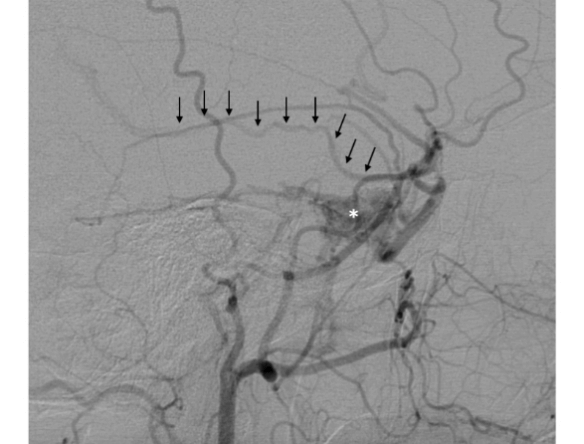
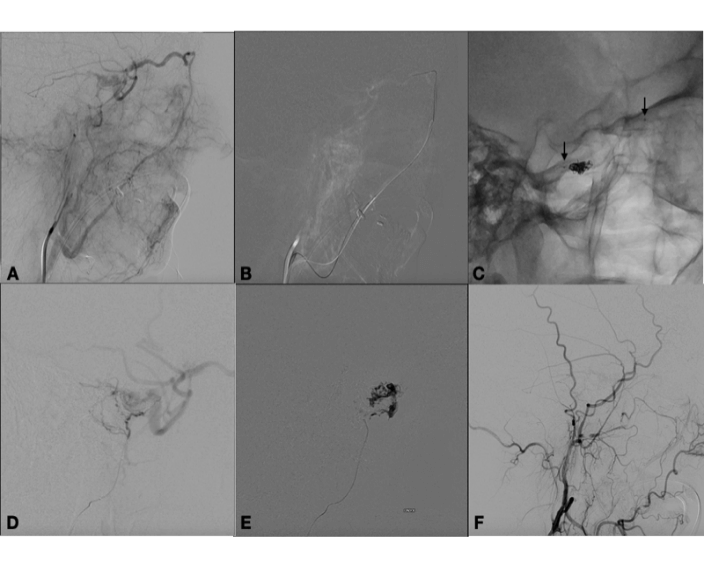
Indeed, diagnostic angiography showed a DAVF of the right cavernous sinus, fed by branches of the right external carotid artery, with drainage to the ipsilateral cavernous sinus and backflow into the superficial middle cerebral vein (Figure 1). Treatment was indicated because of the retrograde flow into the middle cerebral vein, which poses a risk of cerebral haemorrhage. Two weeks after diagnostic angiography, the patient was admitted for treatment. A repeat angiogram showed a change in the fistula pattern, with anterior venous drainage to the superior ophthalmic vein and facial vein. The decision was made to catheterize the fistula via this route and attempt coil and Histoacryl® embolization. Two micro catheters were navigated until de "foot" of the vein, one Magic 1.2 (Balt Extrusion, Montmorency, France), for the glue injection and and one excelsior SL 10 (Stryker Neurovascular, Fremont, California, USA), for coils deployment. First the coils were placed to reduce the flow inside the ophthalmic vein, and after glue was injected. Control angiography showed only partial occlusion of the fistula. The fistula was again catheterized selectively, via the middle meningeal artery, with a sonic microcatheter (Balt Extrusion, Montmorency, France) and an injection of Onyx® liquid embolic system was administered, with complete obliteration of the fistula (Figure 2). Postoperatively, the patient reported right-sided eye pain and slight proptosis. Dexamethasone 4 mg q6h was prescribed, and symptoms had resolved completely by the fourth postoperative day. The patient was asymptomatic on discharge. At 3-month follow-up, she remained free of symptoms and no longer required analgesia.
Figure 1: Selective angiography of the right external carotid artery, in the lateral view, showing a fistula (asterisk) with retrograde drainage into the superficial middle cerebral vein (arrows).
Figure 2: A) Angiography in the venous phase after injection in the external carotid artery showing drainage to the superior ophthalmic vein and facial vein. B) Catheterization of fistula through the facial vein. C) Cast of coils at the take-off of the superior ophthalmic vein and markers of the microcatheter ready for glue injection - arrows. D) Angiography in the lateral view showing microcatheter positioned in the right middle meningeal artery after partial embolization through the superior ophthalmic vein. Note residual filling of the fistula. E) Injection of Onyx® liquid embolic system into the middle meningeal artery. F) Final control angiography showing obliteration of fistula, lateral view.
Discussion
Dural arteriovenous fistulas are acquired lesions which consist of one or more fistulous connections within the leaflets of the dura mater, more specifically involving the walls of a dural venous sinus or adjacent leptomeningeal veins. DAVFs can arise at any age, but manifest predominantly between the fifth and sixth decades of life. There is no gender predilection, except for lesions of the cavernous sinus, 85% of which occur in women. DAVFs are estimated to account for 15% of intracranial arteriovenous malformations. Their pathophysiology is believed to involve venous hypertension; etiologies include traumatic brain injury (TBI), cerebral venous thrombosis, intracranial surgery, and previous infections [1-3].
DAVF of the cavernous sinus is rare, affecting mainly women between the sixth and seventh decades of life. Several classification schemes are available; the most widely used is that proposed by Barrow, which divides cavernous DAVF into 4 types [4-6]:
• Type A – direct shunt between the Internal Carotid Artery (ICA) and the cavernous sinus.
• Type B – shunt between meningeal and cavernous branches of the ICA.
• Type C – shunt between meningeal and cavernous branches of the External Carotid Artery (ECA).
• Type D – shunts between a meningeal branch of the ICA and cavernous branch of the ECA.
Type A fistulas are considered direct; types B, C, and D are considered indirect.
Trigeminal neuralgia affects approximately 0.07% of the population. It is characterized by severe, recurring, sudden, short-term, sharp or electric shock-like pain along one or more trigeminal dermatomes, and is usually unilateral [10]. The etiology and pathophysiology of trigeminal neuralgia are still incompletely understood; however, a neurovascular compression syndrome is found in most patients. Based on etiology, it can be classified into idiopathic, typical or classic (caused by neurovascular compression), or secondary [11].
There are reports of trigeminal neuropathy (not neuralgia) causing symptoms such as paresthesia and facial muscle paresis [6, 7, 9]. Trigeminal neuralgia caused by a cavernous DAVF was first reported by Bartlow et al, Du et al and Fukutome et al [8,12,13]. To the best of our knowledge, corroborated by a literature search, our case is the fourth report worldwide.
Classically, patients with a DAVF of the cavernous sinus present with ocular symptoms, such as chemosis, exophthalmos, eye pain, conjunctival injection, loss of visual acuity, and diplopia, as well as more variable symptoms such as pulsatile tinnitus, headache, and cranial nerve deficits (especially of the abducens nerve, due to its intracavernous course). On physical examination, increased intraocular pressure, ocular bruit, venous congestion, and strabismus may be present. These symptoms occur in fistulas with anterior drainage. In case of posterior drainage, with retrograde flow to the cortical veins, the neurological symptoms correspond to the affected area. Venous infarctions and haemorrhage may occur [14]. Involvement of the trigeminal nerve is rare in both situations. What makes our case even more unusual is the fact that, besides presenting with trigeminal neuralgia, the patient did not have any of the classic ocular signs. Trigeminal disorders have been reported as a possible symptom of cavernous-sinus DAVF; however, they are very uncommon and the mechanism by which the fistula affects the trigeminal nerve is variable, differing across reported cases (Table 1).
Table 1: Reports of carotid-cavernous fistula affecting the trigeminal nerve.
|
Author, year |
Classification |
Cause of symptoms |
Trigeminal syndrome |
Treatment |
Result |
|
Bartlow et al., 1975 [8] |
Direct, high flow (Barrow A) |
Superior petrous sinus dilatation with compression |
Trigeminal neuralgia |
Non reported |
Non reported |
|
von Rad et al., 1975 [7] |
Non reported |
Non reported |
Trigeminal neuropathy |
Carotid artery compression |
Improved |
|
Rizzo et al., 1982 [6] |
Indirect, low flow (Barrow C) |
Gasser ganglion compression or steal of blood flow of the ganglion or venous congestion on Meckel’s cave |
Trigeminal neuropathy |
Surgery |
Cure of tinidus, improved facial parestesia |
|
Du et al., 2003 [12] |
Indirect, high flow (Barrow D) |
Mass efect on gasserian ganglion |
Trigeminal neuralgia |
Embolization |
Absence of pain |
|
Jensen et al., 2004 [9] |
Indirect, low flow (Barrow D) |
Venous congestion on foramen ovale |
Trigeminal neuropathy with difficulty to close the mouth |
Embolization |
Absence of symptoms |
|
Fukutome et al, 2017 [18] |
Indirect, high flow (Barrow D) |
Pulsatile venous compression Meckel cave |
Trigeminal neuralgia |
Embolization |
Absence of pain |
|
Present case |
Indirect, low flow (Barrow B) |
Not identified |
Trigeminal neuralgia |
Embolization |
Absence of pain |
Treatments varied widely across reports. Furthermore, one must bear in mind the evolution of techniques over time and the different presentations of fistulas in different patients. Conventional surgery is in disuse due to the difficulty in accessing the cavernous sinus and its high morbidity. Radiosurgery has occlusion rates that vary between 44-87%, but its biggest limiting factor is the time required for occlusion of the fistula, 6 to 12 months, which can cause worsening of symptoms.
Therefore, the treatment of choice for this disease is endovascular [15]. The endovascular treatment aims to occlude the point of the arteriovenous connection (shunt), and in most cases this is achieved by obliterating the involved segment of the cavernous sinus. Venous access is recommended because it is faster, easier and safer than arterial access. The difficulty of arterial access is due to the fact that the meningeal branches originating in both the external and internal carotid arteries are extremely tortuous and short, which makes navigation and the safe injection of embolic agents difficult. In addition, they present anastomoses with arteries of the cranial nerves and connections with pial branches, which makes the injection of the material more dangerous [16].
The preferred venous access is through the inferior petrosal sinus or the facial vein. Oher routes of access, such as direct puncture of the sinus or catheterization of the superior petrosal sinus, can be used in case classic routes fail [17]. Embolization can be done with coils, liquid embolic agents, or a combination of the two, with cure rates around 85% and low morbidity and mortality. We chose to inject Histoacryl® instead of Onyx® inside the superior ophthalmic vein to avoid creating a mass effect, a complication related when using this last embolic agent [18].
In our case, no single factor was identified that could explain trigeminal involvement. Both MRI and MR angiography failed to show any neurovascular conflict. Cerebral angiography did not identify any findings indicative of direct nerve compression. Nevertheless, endovascular treatment of the fistula was followed by complete remission of symptoms. Fukutome et al. reported a case with possible symptoms due to pulsatile venous compression in Meckel's cave, and maybe this could explain our case as well.
One thing to consider and was very important in this case was to perform angiogram even though MRI and angioMRI were normal. Physicians must be aware that not all vascular diseases are diagnosed by non invasive exames such as MRI or Computer Tomography angiography , and this fact rises up the question if trigeminal neuralgia caused by DAFs is not being under diagnosed.
Conclusion
Trigeminal neuralgia caused by a cavernous-sinus DAVF is a rare entity. The fistula in this case was only diagnosed after digital cerebral angiography was performed, so clinicians must be aware that not all vascular conditions can be identified non invasively, and that cavernous arteriovenous fistulas may be under diagnosed as a possible cause of trigeminal neuralgia.
Article Info
Article Type
Case ReportPublication history
Received: Fri 29, May 2020Accepted: Fri 03, Jul 2020
Published: Sat 11, Jul 2020
Copyright
© 2023 Luciano Manzato. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.07.09
Author Info
Luciano Manzato Paulo M. Mesquita Filho Octavio Karam Victor E. Angeliero Arthur Alberice de Oliveira Luiza Rech Köhler José R. Vanzin
Corresponding Author
Luciano ManzatoInterventional Neuroradiology Department, Neurology and Neurosurgery Service, Passo Fundo, Rio Grande do Sul, Brazil
Figures & Tables
Table 1: Reports of carotid-cavernous fistula affecting the trigeminal nerve.
|
Author, year |
Classification |
Cause of symptoms |
Trigeminal syndrome |
Treatment |
Result |
|
Bartlow et al., 1975 [8] |
Direct, high flow (Barrow A) |
Superior petrous sinus dilatation with compression |
Trigeminal neuralgia |
Non reported |
Non reported |
|
von Rad et al., 1975 [7] |
Non reported |
Non reported |
Trigeminal neuropathy |
Carotid artery compression |
Improved |
|
Rizzo et al., 1982 [6] |
Indirect, low flow (Barrow C) |
Gasser ganglion compression or steal of blood flow of the ganglion or venous congestion on Meckel’s cave |
Trigeminal neuropathy |
Surgery |
Cure of tinidus, improved facial parestesia |
|
Du et al., 2003 [12] |
Indirect, high flow (Barrow D) |
Mass efect on gasserian ganglion |
Trigeminal neuralgia |
Embolization |
Absence of pain |
|
Jensen et al., 2004 [9] |
Indirect, low flow (Barrow D) |
Venous congestion on foramen ovale |
Trigeminal neuropathy with difficulty to close the mouth |
Embolization |
Absence of symptoms |
|
Fukutome et al, 2017 [18] |
Indirect, high flow (Barrow D) |
Pulsatile venous compression Meckel cave |
Trigeminal neuralgia |
Embolization |
Absence of pain |
|
Present case |
Indirect, low flow (Barrow B) |
Not identified |
Trigeminal neuralgia |
Embolization |
Absence of pain |
References
- Lotfi Hacein Bey, Angelos Aristeidis Konstas, John Pile Spellman (2014) Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg 121: 64-75. [Crossref]
- Mohamed Samy Elhammady, Sudheer Ambekar, Roberto C Heros (2017) Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of cerebral dural arteriovenous fistulas. Handb Clin Neurol 143: 99-105. [Crossref]
- A D Henderson, N R Miller (2017) Carotid-cavernous fistula: current concepts in aetiology, investigation, and man- agement. Eye 32: 164-172. [Crossref]
- D L Barrow, R H Spector, I F Braun, J A Landman, S C Tindall et al. (1985) Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg 62: 248-256. [Crossref]
- Matthew David Alexander, Van V Halbach, Danial K Hallam, Daniel L Cooke, Basavaraj Ghodke et al. Relationship of clinical presentation and angiographic findings in patients with indirect cavernous carotid fistulae. J Neurointerv Surg 11: 937-939. [Crossref]
- M Rizzo, E P Bosch, C E Gross (1982) Trigeminal sensory neuropathy due to dural external carotid cavernous sinus fistula. Neurology 32: 89-91. [Crossref]
- M von Rad, K Tornow (1975) Spontane Carotis-cavernosus-Fistel bei Morbus Osler-Rendu. J Neurol 209 237-242. [Crossref]
- B Bartlow, R D Penn (1975) Carotid-cavernous sinus fistula presenting as a posterior fossa mass. J Neurosurg 42: 585-588. [Crossref]
- Robert W Jensen, Hideki Chuman, Jonathan D Trobe, John P Deveikis (2004) Facial and trigeminal neuropathies in cavernous sinus fistulas. J Neuroophthalmol 24: 34-38. [Crossref]
- Giorgio Cruccu (2017) Trigeminal Neuralgia. Continuum 23: 396-420. [Crossref]
- O van Hecke, Sophie K Austin, Rafi A Khan, B H Smith, N Torrance (2014) Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 155: 654-662. [Crossref]
- Rose Du, Devin K Binder, Van Halbach, Nancy Fischbein, Nicholas M Barbaro (2003) Trigeminal Neuralgia in a Patient with a Dural Arteriovenous Fistula in Meckel’s Cave: Case Report. Neurosurgery 53: 216-221. [Crossref]
- Kenji Fukutome, Ichiro Nakagawa, Hun Soo Park, Takeshi Wada, Yasushi Motoyama et al. (2017) Resolution of Trigeminal Neuralgia After Transvenous Embolization of a Cavernous Sinus Dural Arteriovenous Fistula. World Neurosurg 98: 880. [Crossref]
- Jason A Ellis, Hannah Goldstein, E Sander Connolly Jr, Philip M Meyers (2012) Carotid-cavernous fistulas. Neurosurg Focus 32: E9. [Crossref]
- J Zhang, X Lv, C Jiang, Y Li, X Yang, Z Wu (2010) Transarterial and transvenous embolization for cavernous sinus dural arteriovenous fistulae. Interv Neuroradiol 16: 269-277. [Crossref]
- Luís Henrique de Castro-Afonso, Felipe Padovani Trivelato, Marco Túlio Rezende, Alexandre Cordeiro Ulhôa, Guilherme Seizem Nakiri et al. (2019) The routes for embolization of dural carotid cavernous fistulas when the endovascular approach is indicated as a first-line strategy. Interv Neuroradiol 25: 66-70. [Crossref]
- Felipe Padovani Trivelato, Luciano Bambini Manzato, Paulo Moacir Mesquita Filho, Alexandre Cordeiro Ulhôa, José Ricardo Vanzin et al. (2018) Transorbital Cavernous Sinus Direct Puncture: Alternative to treat dural arteriovenous fistula. Clin Neuroradiol 28: 55-61. [Crossref]
- Luís Henrique de Castro-Afonso, Felipe Padovani Trivelato, Marco Túlio Rezende, Alexandre Cordeiro Ulhôa, Guilherme Seizem Nakiri et al. (2018) Transvenous embolization of dural carotid cavernous fistulas: the role of liquid embolic agents in association with coils on patient outcomes. J Neurointerv Surg 10: 461-462. [Crossref]
