Subcutaneous Implantable Cardioverter Defibrillator and LifeVest Saving Lives in Challenging Scenarios
Subcutaneous Implantable Cardioverter Defibrillator and LifeVest Saving Lives in Challenging Scenarios
A B S T R A C T
Introduction: According to guidelines, ICD should be implanted in selected patients for primary or secondary prevention. The increasing number of procedures leads to a growth of infective complications.
Case Summary: We report two cases of ICD implantation complicated with severe infections. The first procedure was complicated by endocarditis and septic shock, the second one by septic shock. After antibiotics and ICD explantation, a wearable cardioverter defibrillator (WCD) was used as a bridge to subcutaneous ICD (S-ICD).
Conclusion: These two cases demonstrate the benefits of the S-ICD, but also the chance to treat high-risk patients with WCD as bridge-to-procedure, allowing full recovery before a new implantation.
Keywords
Subcutaneous ICD, infective complications, septic shock, wearable cardioverter defibrillator, case report
Introduction
According to guidelines, implantable cardioverter defibrillators (ICD) should be considered for secondary prevention when a patient experimented an episode of ventricular fibrillation or sustained ventricular tachycardia not related to reversible causes, or for primary prevention when left ventricular ejection fraction (LVEF) is severely reduced (< 35%) after at least 3 months of optimized medical therapy in the setting of ischaemic or nonischaemic cardiomyopathies [1, 2]. In patients with hypertrophic cardiomyopathy (HCM), ICD can be implanted on the base of a risk score of sudden cardiac death (SCD) that takes into consideration various aspects [3].
The increasing number of implantation procedures leads to a growth of related complications, as pocket or lead infections, endocarditis or even septic shock [4]. The treatment of choice for these infections is the removal of the entire system, together with antimicrobial therapy [5]. These patients, after recovering, maintain a high infective risk, together with the need for persistent ICD protection. Current guidelines suggest in these complex scenarios the use of a wearable cardioverter defibrillator (WCD) as a bridge to a new implant [6]. Moreover, subcutaneous ICD (S-ICD) could be considered in such patients as a long-term strategy of systemic infection and endocarditis prevention, thanks to the absence of endocavitarian leads [7]. We present two cases of severe and life-threatening ICD implantation related infections, treated with WCD as a bridge to subsequent S-ICD.
Case 1
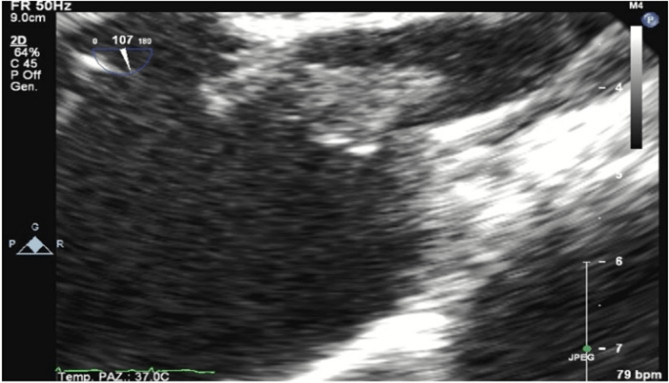
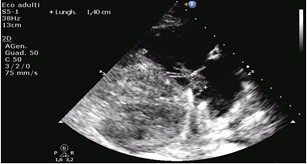
A 15-year-old boy with HCM came to the emergency department (ED) of our hospital complaining fever started about 15 days after endocavitarian dual-chamber ICD implantation in another center for primary prevention. Of note, after three days from the procedure, he complained of fever and left shoulder pain next to the device pocket. Empirical antimicrobial therapy with amoxicillin was started and maintained for 7 days, without complete regression of the symptoms. At that time, the pocket was evaluated, and it did not show any sign of infection, and a transthoracic echocardiogram was negative for vegetations or abscesses. In the ED, the boy was drowsy, tachycardic and dyspnoeic. His temperature was 40°C, blood pressure was 80/45 mmHg, and the heart rate was 140/min. A systolic murmur was hearable at cardiac evaluation, there were no signs of pulmonary or peripheric congestion and no signs of pocket infection. The laboratory test showed C-reactive protein (CRP) of 300 mg/L (n.v. 0-5 mg/L), white blood cells of 9090/mm3 (8000/mm3 neutrophils, 450/mm3 lymphocytes), hemoglobin 12,3 g/dl, platelets 32000/mm3. Transthoracic and transoesophageal echocardiograms were performed, showing double vegetation adhere to the anterior and posterior leaflet of the tricuspid valve and on the chordae, with mild regurgitation (Figures 1 & 2). A total body computed tomography (CT) was also performed, showing multiple pulmonary abscesses.
Figure 1: Patient 1: Transesophageal echocardiography showing the tricuspid vegetation.
Figure 2: Patient 1: Echocardiographic measurement of the vegetation.
In the strong suspicion of endocarditis complicated with septic shock and peripherical embolizations, broad spectrum antibiotics were started (daptomycin, gentamicin and meropenem) and the patient was transferred to the intensive care unit. Also, considering the clinical state a consequence of the ICD implantation, the device, together with the catheters, were safely removed and all the system was sent to microbiology for cultural exams. Blood cultures were positive for multi-sensitive S. aureus, and the same results were obtained on the sonication of the ICD generator, on the cultural examination of the catheters, and on the tampon of the pocket. The antibiotic therapy was fixed according to antibiograms, and meropenem was suspended. After two weeks, the patient was referred to the cardiac-surgery team, and a tricuspid valve plastic was performed, with complete removal of the vegetations. Considering the persistent indication to ICD therapy, even if in primary prevention and in a patient with a major life-threatening infection, we decided to candidate the patient to four months of WCD wearing (LifeVest, ZOLL Medical Corporation, Chelmsford, Massachusetts, USA), allowing him to fully recover from the endocarditis before a new ICD could be definitely implanted. He lived with LifeVest for 119 days, with excellent compliance (23.78 hours/day of wearing). After that period, the patient was implanted with S-ICD (Emblem; Boston Scientific Corporation, St. Paul, Minnesota, USA) without any complication. In the following 30 months, the patient performed regular clinical and device follow up, he remained asymptomatic in the absence of ICD interventions, and there were no infectious relapses.
Case 2
A 58-year-old man with dilated cardiomyopathy associated with advanced functional class and severe left ventricular dysfunction despite optimal medical therapy was admitted to the Cardiology Unit to be implanted with an ICD in the setting of primary prevention. Other comorbidities were hypertension, permanent atrial fibrillation, recent surgery for bladder cancer, and hemodyalitic chronic renal failure (via right central venous Tesium catheter). An endocavitarian ICD was implanted without complications, pre-medicating the patient with cefazolin before the procedure. Immediately after the end of the procedure, the patient started complaining of fever (39°C), shivers and dyspnea. His blood pressure fell (70/40 mmHg), and he was tachycardic (150/min). An arterial blood gas analysis showed lactic acidosis with initial hypoxia. Oxygen therapy and intravenous hydration were started, together with inotropes and vasopressor, to sustain a favorable hemodynamic status. Laboratory tests showed CRP 150 mg/L, procalcitonin 43 ng/ml (n.v.< 0,1 ng/ml), WBC 7600/mm3, hemoglobin 10,9 g/dl; platelets 98000/mm3.
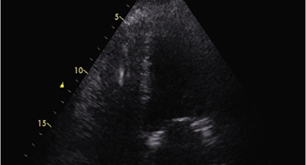
Figure 3: Patient 2: Transthoracic echocardiography showing ICD right ventricular catheter without signs of infection (imaging during sepsis).
The patient was then intubated considering the progressive deterioration of the hemodynamic conditions, and in the suspicion of septic shock, antimicrobial therapy was started (high ev doses of piperacillin/tazobactam plus daptomycin). A transthoracic echocardiogram was performed, showing severe left ventricular disfunction without pericardial effusion. No signs of vegetations of abscesses (Figure 3). Blood cultures were positive for Serratia marcescens resistant to ampicillin/sulbactam plus piperacillin/tazobactam, so the antibiotic therapy was rearranged (switch to ceftazidime and amikacin). The ICD system and the Tesium catheter were both removed and sent to microbiology, but no organism grew up. The patient gradually recovered, he was extubated, and the inotropes were gradually set down. Considering the patient’s conditions, the persisting indication of an ICD, and the recent major infective insult, we decided to candidate the patient for three months of WCD (LifeVest), allowing him to recover fully before a new ICD could be definitely implanted. He lived with LifeVest for 118 days, with excellent compliance (23.95 hours/day of wearing). After that period, the patient was implanted with an S-ICD (Emblem Boston) without any complication. The subsequent follow up was free of complications, without infectious relapses. The patient is free of ICD interventions by now, and a new Tesium catheter was placed because of the inefficiency of an arterio-venous fistula recently made up.
Discussion
Pacemaker and ICD implantations, together with implant revisions and generators or leads substitution, greatly increased during the last few years [8]. The increasing number of implantation procedures leads to a growth of people who experimented complications directly derived from them as pocket infections, lead infections, endocarditis, or even septic shock. The more procedures a patient undergoes, the higher the risk of infective complications. These are two emblematic cases of primary prevention ICD implantation complicated with severe infections. Of note, in the first case, cultural examinations of the leads and sonication of the generator shared the same germ that was found in the blood stream. In the second case, the generator and lead were bacteria-free, likely because of the broad-spectrum antimicrobial therapy started 3 days before the system explantation. Of note, infections are the main cause of ICD generator and lead extraction [5]. The incidence of device infections varies from 0.5 to 2.2%, with a 6% mortality rate in two years. If patients show signs of systemic infections or sepsis, the mortality rate reaches almost 15-25%. Associated negative factors are advanced age, comorbidities and device complexity [4].
After a major infective event, indications on implantation of devices could be revised accordingly on patient conditions and arrhythmic risk [6]. Balancing the patients’ conditions, the fragility after the major infective insult, and the persisting indication of ICD implantation, we decided to candidate them to four months of external WCD, a null-invasive but highly protective anti-arrhythmic device, allowing them to recover fully before a new ICD could be definitely implanted. The use of WCD in such cases is considered and expected by recent guidelines [6]. In that period, our patients did not experience WCD interventions, and they were also free of major arrhythmic events after S-ICD implantation. After four months of WCD prevention, they completely and successfully recovered; therefore, we decided to protect them with a subcutaneous ICD, which is a less invasive but highly effective device [7]. Both patients underwent a free-of-complications clinical course.
Conclusion
In these two high-risk patients, we demonstrated the efficacy of early application of WCD after ICD removal for life-threatening infections, and the benefits of subsequent S-ICD implantation.
Consent
Our patients signed informed consent form for the material presented to appear in the publication above and in related publications, prior to submission.
Acknowledgements
We would like to thank Boston Scientific Corporation Eng. Carlo Rojatti and Eng. Paolo Cigagna for their precious support and data.
Funding
The study was funded by Boston Scientific Corporation.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Thu 27, Aug 2020Accepted: Fri 11, Sep 2020
Published: Thu 12, Nov 2020
Copyright
© 2023 Domenico Facchin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.03.03
Author Info
Domenico Facchin Tioni C Daleffe E Rebellato L Antonutti M Sut D Proclemer A
Corresponding Author
Domenico FacchinCardiology Division, Department of Cardiothoracic Science, Azienda Sanitaria Universitaria Friuli Centrale Santa Maria della Misericordia, Udine, Italy
Figures & Tables
References
- Silvia G Priori, Carina Blomström Lundqvist, Andrea Mazzanti, Nico Blom, Martin Borggrefe et al. (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36: 2793-2867. [Crossref]
- Sana M Al Khatib, William G Stevenson, Michael J Ackerman, William J Bryant, David J Callans et al. (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm. Heart Rhythm 15: e190-e252. [Crossref]
- Perry M Elliott, Aris Anastasakis, Michael A Borger, Martin Borggrefe, Franco Cecchi et al. (2014) 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35: 2733-2779. [Crossref]
- Grzegorz Sławiński, Ewa Lewicka, Maciej Kempa, Szymon Budrejko, Grzegorz Raczak (2019) Infections of cardiac implantable electronic devices: epidemiology, classification, treatment and prognosis. Adv Clin Exp Med. 28: 263-270. [Crossref]
- Federico Migliore, Martina Testolina, Antonio Sagone, Domenico Carretta, Tullio Agricola et al. (2019) Multicenter experience with the Evolution RL mechanical sheath for lead extraction using a stepwise approach: Safety, effectiveness, and outcome. Pacing Clin Electrophysiol 42: 989-997. [Crossref]
- Ahmad Masri, Ahmed M Altibi, Sebhat Erqou, Mohammad A Zmaili, Ala Saleh et al. (2019) Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol 5: 152-161. [Crossref]
- Antonio D'Onofrio, Paolo Pieragnoli, Mauro Biffi, Gerardo Nigro, Federico Migliore et al. (2018) Subcutaneous implantable cardioverter defibrillator implantation: An analysis of Italian clinical practice and its evolution. Int J Cardiol 272: 162-167. [Crossref]
- Stefano Poli, Giuseppe Boriani, Massimo Zecchin, Domenico Facchin, Maurizio Gasparini et al. (2019) Favorable Trend of Implantable Cardioverter-Defibrillator Service Life in a Large Single-Nation Population: Insights From 10-Year Analysis of the Italian Implantable Cardioverter-Defibrillator Registry. J Am Heart Assoc 8: e012759. [Crossref]
