Journals
Significance of Body Composition and Systemic Inflammation in Patients with Operable Colon Cancer Treated with Curative Intent
A B S T R A C T
Background: Muscle abnormalities and systemic inflammation have been associated with cancer progression and poor disease outcomes in patients with colon cancer. These factors are easily evaluated and can potentially be modified to improve outcomes. The objective of this study is to investigate the relationship between computed tomography (CT) derived measures of body composition, including low muscle mass (sarcopenia) and low muscle radiodensity (myosteatosis). It will also examine their association with systemic inflammation and determine whether these factors impact hospital length of stay for patients undergoing resection of their primary colorectal cancer.
Methods: This study included 133 patients with stage I to III colon cancers diagnosed from 2011 through 2018 who underwent resection with curative intent. CT scans were used to identify sarcopenia and myosteatosis using predefined sex-specific and body mass index (BMI)-specific thresholds. The primary measure for systemic inflammation was the neutrophil-to-lymphocyte ratio. Tumour and patient characteristics were recorded. The primary outcome was hospital length of stay. Associations between body composition and systemic inflammation were examined using linear regression analyses, and their relationship with post-surgical length of stay was determined using logistic regression analyses.
Results: A significant proportion of patients were overweight or obese (60.9%). Sarcopenia and myosteatosis were highly prevalent (41.4% and 39.1% respectively). Muscle mass and muscle radiodensity were not significantly correlated with each other. Male sex (p < 0.001) and higher BMI (p < 0.001) were associated with greater muscle mass. Male sex (p = 0.020) was also associated with greater muscle radiodensity but higher BMI (p < 0.001) was associated with lower muscle radiodensity. Inflammation was present in 39.1% of patients. Elevated neutrophil-to-lymphocyte ratio was associated with longer length of stay (OR 1.29, 95% CI 1.04-1.61, p = 0.019).
Conclusion: Sarcopenia and myosteatosis were prevalent among colon cancer patients, despite many of them being overweight or obese. Systemic inflammation was associated with prolonged length of stay post-surgery and could potentially be utilised to delineate patients with poorer recovery and who may benefit from additional monitoring or interventions to reduce the length of hospitalisation. These commonly collected markers could enhance prognostication and identify patients with a poorer outcome.
Keywords
Colon cancer, Computed tomography, Body composition, Sarcopenia, Myosteatosis, Body mass index, Systemic inflammation, Neutrophil-to-lymphocyte ratio
Introduction
In Australia, colorectal cancer (CRC) was the third most commonly diagnosed cancer in 2014, and it was the second leading cause of cancer death in 2016, with a five-year survival rate of 69% [1]. Despite current prognostic stratification systems, clinical outcomes still vary considerably among patients with the same characteristics and stage of disease. Identifying additional factors that can predict patients at high risk of adverse disease outcomes and premature mortality is a clinical priority. Increasing number of studies have examined body composition and systemic inflammation as novel prognostic indicators [2, 3].
Recent studies that have examined associations between markers of body composition and cancer outcomes have suggested that muscle abnormalities characterised by sarcopenia and myosteatosis were associated with poorer cancer prognosis in general and more specifically in patients with CRC [4-6]. The term sarcopenia was first mentioned by Baumgartner et al. to describe the age-related loss of muscle mass observed in the elderly population [7]. Subsequent studies have also demonstrated its prevalence in patients with chronic diseases and cancer [8]. More recently, the definition of sarcopenia has been adapted in oncological settings as excessive loss of skeletal muscle mass and has been associated with adverse outcomes. In particular, it predicts poor postoperative and surgical outcomes, treatment toxicities, and reduced survival [5, 9-13]. Myosteatosis refers to inter- and intramuscular fatty infiltration, and it is also a normal by-product of aging, but it can be exacerbated by disease and therefore is common in patients with cancer [6]. Studies have shown that it is associated with postsurgical complications and elevated risks of mortality [6, 14]. As the world prevalence of overweight and obesity continues to rise, these muscle abnormalities may be masked by traditional body composition measures such as body mass index (BMI). Routine diagnostic imaging using computed tomography (CT) allows accurate quantification of skeletal muscle in patients of various BMI, revealing otherwise occult muscle depletion. Similarly, systemic inflammation has also been suggested to play an essential role in cancer progression and has been reported to be an indicator of poor prognosis in several malignancies [3]. Pre-treatment values of neutrophil-to-lymphocyte ratio (NLR) is one standard measure of systemic inflammation and have been suggested to predict surgical complications and survival [15, 16]. Since measures of body composition and systemic inflammation can be evaluated with existing clinical data, they may become new indicators to identify patients with poorer prognosis.
Colon cancers are conventionally grouped with rectal cancers and collectively referred to as CRC. Nonetheless, rectal cancers can be considered a distinct disease from colon cancers. Apart from anatomical differences, the survival of rectal cancer patients is also influenced by neoadjuvant or adjuvant radiotherapy, rendering the analysis of outcomes more complex. Whereas body composition and systemic inflammation have been studied extensively in the CRC population, to the best of our knowledge, no prior studies have examined the relationship of body composition and systemic inflammation and their independent associations with colon (excluding rectal) cancer outcomes. Not only are these factors important as promising prognostic indicators, but they are also potentially modifiable. Therefore, the present study aimed to examine the relationship between CT measures of body composition and systemic inflammation and their associations with short-term outcomes in patients with primary colon cancer.
Materials and methods
I Study population
This retrospective study identified all patients diagnosed with stage I to III colon cancer at St Vincent’s Hospital Melbourne (SVHM) between December 2011 and July 2018 who underwent surgical resection with curative intent (n = 286). Patients with a preoperative abdominal CT scan, recorded height and weight measurements and pre-treatment NLR available from clinically acquired laboratory data were included. Patients who had rectal cancer or with metastatic disease from other cancer types were not considered for inclusion. 35 patients who received a diagnosis of rectal cancer were excluded. We also excluded 84 patients who did not have an electronically available abdominal CT scan at SVHM and 19 patients without valid height and weight measures. Exclusion also included 15 patients who had metastatic disease from other cancer types. This left 133 patients in the final analytic sample. The study was approved by the SVHM Ethics Review Board. Informed consent was waived due to the retrospective nature of the study.
II Body composition assessment
Preoperative digital CT scans were used to quantify measures of body composition. Two consecutive images at the level of the third lumbar (L3) vertebrae were identified to assess skeletal muscle area and muscle radiodensity according to predefined tissue-specific Hounsfield Units (HU) ranges (–29 to +150) using Slice-O-Matic Software (version 5.0; TomoVision). Abdominal cross-sectional areas at the L3 vertebrae are strongly correlated with whole body volumes of muscle mass and adipose tissue and its use have been validated in oncological settings [17, 18]. Skeletal muscle areas included rectus abdominus, erector spinae muscles, quadratus lumborum, psoas, and internal, transverse and external oblique muscle groups.
Cross-sectional muscle surface area values in centimetres squared (cm2) at the L3 vertebrae were obtained, and the mean muscle area was normalised for height in metres (m2). This was reported as the skeletal muscle index (SMI, cm2/m2). Sex-specific and BMI-specific cut-offs for SMI were then selected from previously published studies to define sarcopenia: for normal or overweight patients (BMI < 30), these were less than 52 cm2/m2 for men and less than 38 cm2/m2 for women, while for obese patients (BMI ≥ 30), these were less than 54 cm2/m2 for men and less than 47 cm2/m2 for women13. Skeletal muscle radiodensity (SMD, HU) was derived from the mean muscle radiodensity (HU) measured for the entire cross-sectional muscle area from the same images used to calculate SMI. Sex-specific cut-offs for SMD were then selected from previously published studies to define myosteatosis: for female patients, this was less than 32.5 HU, and for male patients, this was less than 35.5 HU [6].
III Systemic inflammation assessment
The primary measure of systemic inflammation in this study was NLR from laboratory values obtained as part of routine blood tests. Pre-treatment haematological markers were recorded, and NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The preoperative NLR closest to the time of surgery was selected. NLR values greater than or equal to 3 were considered elevated [13].
IV Covariate assessment and endpoints
SVHM electronic medical records were reviewed to obtain information on potential prognostic and confounding variables, including patient demographics (age and sex), cancer stage, tumour characteristics, surgical and treatment information. Height and weight measurements closest to the time of cancer diagnosis were selected to calculate BMI. Patients were classified as underweight (BMI < 18.5), normal weight (BMI ≥ 18.5 to < 25), overweight (BMI ≥ 25 to < 30) or obese (BMI > 30). Length of stay (LOS) was considered from the day of surgery to the day of discharge.
V Statistical analysis
In exploratory analyses, descriptive statistics were tabulated (mean [SD], median [interquartile, 25-75% range] and percentages). Next, the inter-relationship between sarcopenia and myosteatosis was examined using Spearman’s correlation coefficients. A correlation was considered to be weak with coefficient values of < 0.200 and strong with values > 0.800. Lastly, the relationship between baseline characteristics and CT measures of body composition as well as the inter-relationship between CT measures of body composition and systemic inflammation were examined using univariate and multivariate linear regression analyses, adjusting for covariates including age at diagnosis, sex, cancer stage and BMI.
To enable us to determine univariate and multivariate predictors for a delayed discharge, median LOS was calculated. Patients with a LOS above the median were classified into the “long” group while those patients with a LOS below the median were classified into the “short” group. The independent associations of baseline characteristics, body composition and systemic inflammation with LOS were then examined using univariate and multivariate logistic regression analyses. All statistical analyses were performed using Stata (version 15.1; StataCorp LLC). Statistical significance was established with p-value < 0.050.
Results
I Baseline characteristics
Table 1 summarises the characteristics of 133 patients with stage I to III colon cancer. Most patients were over 65 years of age (66.9%) and overweight or obese (60.9%). Of the 133 patients, 55 patients (41.4%) were classified as having sarcopenia (regardless of myosteatosis status), and 52 patients (39.1%) were classified as having myosteatosis (regardless of sarcopenia status). A third group consisting of 21 patients (15.8%) who had both sarcopenia and myosteatosis was also noted. 52 patients (39.1%) were identified with underlying inflammation (NLR ≥ 3).
Table 1: Baseline characteristics of the study population.
|
Characteristic |
n = 133 |
|
Age (years) |
69.1 ± 13.8 |
|
Sex |
|
|
Male |
75 (56.4%) |
|
Female |
58 (43.6%) |
|
Cancer stage |
|
|
I |
35 (26.3%) |
|
II |
52 (39.1%) |
|
III |
46 (34.6%) |
|
BMI category |
|
|
Underweight |
4 (3.0%) |
|
Normal |
48 (36.1%) |
|
Overweight |
38 (28.6%) |
|
Obese |
43 (32.3%) |
|
Sarcopenia status |
|
|
Yes |
55 (41.4%) |
|
No |
78 (58.6%) |
|
Myosteatosis status |
|
|
Yes |
52 (39.1%) |
|
No |
81 (60.9%) |
|
Body composition measures |
|
|
BMI (kg/m2) |
26.6 (23.2 - 31.2) |
|
SMI (cm2/m2) |
48.0 (43.0 - 54.8) |
|
SMD (HU) |
38.3 (29.9 - 47.9) |
|
Inflammation status |
|
|
NLR ≥ 3 |
52 (39.1%) |
|
NLR < 3 |
81 (60.9%) |
|
Inflammation measure |
|
|
NLR |
2.6 (1.9 - 4.1) |
|
LOS (days) |
7 (5 - 12) |
II Patient characteristics and CT derived measures of body composition
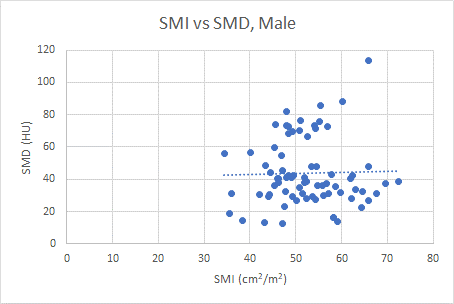
There was no correlation between SMI and SMD, regardless of sex (Figure 1). Males tended to be more muscular (mean SMI 52.4 ± 8.1 vs. 44.7 ± 6.9 cm2/m2) and had higher muscle radiodensity (mean HU 43.8 ± 20.6 vs. 38.3 ± 13.6 HU) than females. We also noted that a higher proportion of underweight and normal weight patients (53.8%) were sarcopenic compared to overweight and obese patients (33.3%), whereas myosteatosis seemed to be more common in overweight and obese patients (51.9%) versus those who were underweight and normal weight (19.2%). Patients who were sarcopenic had lower BMI (26.1 ± 5.6 vs. 28.9 ± 6.7) compared to patients with normal muscle mass. Patients with myosteatosis had a higher BMI (31.4 ± 7.2 vs. 25.4 ± 4.6) compared to patients with normal muscle radiodensity. Despite these trends, sarcopenia and myosteatosis can occur in patients across all BMI categories (Figure 2).
These initial findings were confirmed on subsequent regression analyses (Table 2). On univariate analysis, there was an association between male sex (p < 0.001) and increased BMI (p < 0.001) with higher SMI. On multivariate analysis, male sex (p < 0.001) and increased BMI (p < 0.001) remained significantly associated with higher SMI. Of note, there was no association between age, cancer stage and SMI. On univariate analysis, there was an association between increased BMI (p < 0.001) and lower SMD. On multivariate analysis, increased BMI (p < 0.001) remained significantly associated with lower SMD and male sex (p = 0.020) was associated with higher SMD. Again, there was no association between age, cancer stage and SMD.
Figure 1: Scatterplot illustrating the association of SMI and SMD, in males and females. Abbreviations: SMI, skeletal muscle index; SMD, skeletal muscle radiodensity.
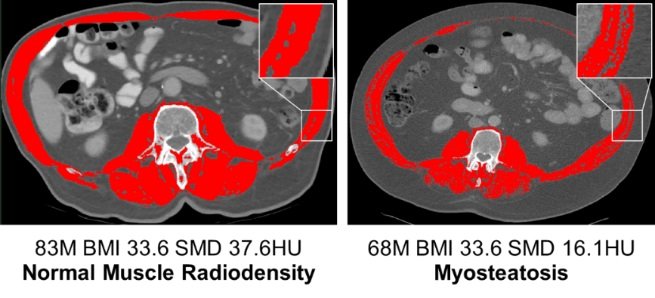
Figure 2: Representative cross-sectional CT images from patient with normal muscle mass, patient with sarcopenia, patient with normal muscle radiodensity and patient with myosteatosis. The images are cross-sectional CT scans at the L3 level with skeletal muscle highlighted in red. The two images on the top row show variation in SMI for overweight female patients with identical BMI. Patient on the top left had normal muscle mass, whereas patient on the top right meets the criteria for sarcopenia. The two images at the bottom row show variation in SMD for obese male patients with identical BMI. Patient on the bottom left had normal muscle radiodensity, whereas patient on the bottom right meets the criteria for myosteatosis. Abbreviations: CT, computed tomography; BMI, body mass index; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity.
III Patient characteristics, CT derived measures of body composition and systemic inflammation
We were unable to identify any patient characteristics or clinical features that predicted inflammation. On univariate and multivariate analyses, there were no significant associations between age, sex, cancer stage and BMI with NLR. We noted that a higher proportion of patients with inflammation (55.8%) were sarcopenic compared to those without inflammation (32.1%). Indeed, on univariate analysis, there was an association between elevated NLR (p = 0.014) and lower SMI. However, on multivariate analysis (Table 2), NLR (p = 0.089) was no longer significantly associated with SMI. Conversely, myosteatosis was more common in patients without inflammation (45.7%) versus those with inflammation (28.8%). This was supported by findings from regression analyses. On univariate analysis, there was an association between elevated NLR (p = 0.017) and greater SMD. Elevated NLR (p = 0.050) remained associated with greater SMD on multivariate analysis (Table 2).
IV Body composition, systemic inflammation and LOS
The median LOS for patients undergoing curative resection was 7 (range 5-12) days. Sarcopenia and myosteatosis did not associate with any significant changes in LOS. Median LOS in patients who were sarcopenic was 8 (range 5-13) days compared to 6 (range 5-9) days in those who did not have sarcopenia. Similarly, patients with myosteatosis had a median LOS of 8 (range 5-10) days compared to those without myosteatosis who had a median LOS of 7 (range 5-12) days. Indeed, on univariate and multivariate analyses (Table 3), there were no associations between SMI and SMD with LOS. On the other hand, inflammation seemed to predict prolonged LOS. In patients with NLR of 3 or greater, the median LOS was 8 (range 5-12) days in comparison to patients with NLR less than 3 who had a median LOS of 6 (range 5-9) days. This was validated in regression analyses (Table 3). On univariate analysis, there was an association between increased age (odds ratio [OR] 1.03, p = 0.036) and elevated NLR (OR 1.28, p = 0.014) with longer LOS. On multivariate analysis, increased age (OR 1.03, p = 0.022) and elevated NLR (OR 1.29, p = 0.019) remained significantly associated with longer LOS.
Table 2: The relationship between patient characteristics, cancer stage, BMI, NLR and measures of body composition.
|
|
Variable |
Coefficient |
95% CI |
P-value |
|
SMI |
|
|
|
|
|
|
Age |
–0.05 |
-0.13 - 0.03 |
0.181 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
7.39 |
5.18 - 9.60 |
< 0.001 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
0.50 |
–2.32 - 3.33 |
0.725 |
|
|
III |
1.20 |
–1.61 - 4.02 |
0.399 |
|
|
BMI |
0.64 |
0.47 - 0.81 |
< 0.001 |
|
|
NLR |
–0.50 |
–1.08 - 0.08 |
0.089 |
|
SMD |
|
|
|
|
|
|
Age |
–0.20 |
–0.41 - 0.01 |
0.057 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
6.92 |
1.13 - 12.72 |
0.020 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
5.91 |
–1.50 - 13.31 |
0.117 |
|
|
III |
1.01 |
–6.36 - 8.38 |
0.787 |
|
|
BMI |
–0.88 |
–1.32 - –0.43 |
< 0.001 |
|
|
NLR |
1.52 |
–0.00 - 3.03 |
0.050 |
Multivariate linear regression analyses for body composition measures. Abbreviations: BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity; CI, confidence interval.
Table 3: The relationship between patient characteristics, cancer stage, BMI, measures of body composition, NLR and LOS.
|
|
Variable |
OR |
95% CI |
P-value |
|
|
Age |
1.03 |
1.00 - 1.07 |
0.022 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
0.85 |
0.36 - 2.03 |
0.714 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
1.27 |
0.49 - 3.30 |
0.627 |
|
|
III |
0.86 |
0.33 - 2.22 |
0.751 |
|
|
BMI |
1.00 |
0.93 - 1.08 |
0.929 |
|
|
SMI |
1.00 |
0.94 - 1.06 |
0.994 |
|
|
SMD |
1.00 |
0.98 - 1.03 |
0.800 |
|
|
NLR |
1.29 |
1.04 - 1.61 |
0.019 |
Multivariate logistic regression analysis for LOS. Abbreviations: BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio; LOS, length of stay; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity; OR, odds ratio; CI, confidence interval.
Discussion
Our objective was to identify the relationship between sarcopenia and myosteatosis, to examine the association of these muscle abnormalities with systemic inflammation, and to investigate if these disease states impact LOS in patients with non-metastatic colon cancer who underwent surgical resection with curative intent. To our knowledge, this is the first study to examine the relationships between CT derived measures of body composition and markers of systemic inflammation and their independent associations with LOS in a cohort comprised entirely of colon excluding rectal cancer patients. In our cohort of 133 patients with stage I to III colon cancer, we found that SMI was not correlated with SMD, elevated NLR was associated with higher SMD at diagnosis, and that elevated NLR was associated with a longer LOS.
CRC has been extensively examined with reference to CT derived body composition measures. However, most studies have focused on either SMI or SMD. Few studies have included both measurements in their analysis and the relationship between sarcopenia and myosteatosis is not well understood. Dolan et al. in their study consisting of 650 patients with primary operable CRC demonstrated only a weak association between SMI and SMD [19]. In contrast, our study showed no correlation between SMI and SMD. In fact, sarcopenia and myosteatosis do not frequently occur simultaneously, and their associated clinical factors are not the same. This may suggest that sarcopenia and myosteatosis are independent muscle abnormalities that represent two separate biological processes leading to muscle depletion. One possibility is that sarcopenia and myosteatosis are driven by different chemical and molecular factors released from the tumour. Another explanation is that there may be differences in host susceptibility resulting in different manifestations of muscle depletion. In addition, several other studies also demonstrated independent effects of sarcopenia and myosteatosis on survival outcomes, as well as an additive effect of these two muscle abnormalities with a particularly poor prognosis [4, 6]. Therefore, the incorporation of both types of body composition measures into the evaluation of muscle health is critical and can provide valuable information.
Sarcopenia and myosteatosis were prevalent in our patient cohort. Sarcopenia was found in 41.4% and myosteatosis in 39.1% of patients, despite the wide BMI range. We found that BMI was positively related to SMI, which is consistent with previous studies across cancer types [4]. Muscle mass may increase concurrently with the increase in adipose tissue for most patients, which may partially explain the lower risk of sarcopenia in patients with higher BMI in our cohort. Despite this lower risk, the prevalence of sarcopenia was 20.3% in patients who were overweight or obese, and the concurrence of sarcopenia and obesity was prevalent at 12.0%. These patients, as described by Baracos and Arribas, possess a unique body habitus termed sarcopenic obesity where they are simultaneously in the highest ranges of adipose mass and the lowest ranges of muscularity [20]. They represent a group of individuals where significant changes in body composition occur but are not always recognisable or detected by physical appearance or BMI [21]. We also noted that BMI was negatively related to SMD. The negative relationship between high BMI and low SMD is consistent with a previous study comprising of 3262 stage I to III invasive CRC patients [22]. The precise mechanism leading to SMD decline in cancer has not been determined, but it is reasonable to speculate that ectopic fat infiltrates into skeletal muscle with advanced age, resulting in the manifestation of low SMD [22]. Because BMI cannot distinguish muscle from fat, patients with sarcopenic obesity or other body composition phenotypes often go undetected. These observations may have profound implications for the treatment of sarcopenia and myosteatosis in patients with colon cancer, and potentially other common solid tumours. The future use of CT scans to identify sarcopenia and myosteatosis will facilitate the identification of those at risk and will be useful for assessing the impact of targeted interventions.
Body composition and systemic inflammation have previously been associated with prognosis in CRC and other cancers, but most studies have addressed them individually. More recently, a few studies have explored the relationship between body composition measures and systemic inflammation [13, 23-25]. In those studies that included NLR as a measure of the systemic inflammatory response, they have consistently demonstrated that systemic inflammation was associated with lower SMI and SMD [13, 23]. For example, in a cross-sectional study of 763 patients with stage I to IV CRC, Malietzis et al. found that elevated preoperative NLR was associated with compromised muscle mass and quality [23]. Cespedes Feliciano et al. in their recent study of 2470 stage I to III CRC also reported that higher NLR in the months prior to diagnosis was associated with sarcopenia at diagnosis [13]. However, our results showed conflicting findings. Firstly, in our study, elevated NLR was associated with higher SMD rather than lower SMD at diagnosis. The reason for our finding is unclear. It may be that individuals with healthier muscle quality in our cohort had acute inflammatory conditions such as sepsis before surgery that were not accounted for in this study, which contributed to the higher NLR values. However, there is also the possibility that our discordant finding was simply due to the small sample size of our study. Future studies should incorporate these factors into consideration to better delineate the relationship between myosteatosis and systemic inflammation in a larger study cohort. Secondly, our study established only an association between SMI and NLR on univariate analysis but not when adjusted for covariates. A larger study population in the future could improve the characterisation of this relationship.
Since sarcopenia and myosteatosis in the context of cancer are thought to represent pathological muscle depletion, it is reasonable that recovery from surgery would be impaired, particularly if there is consequent impaired wound healing or development of complications after surgery, leading to prolonged hospitalisation. However, we did not observe sarcopenia and myosteatosis being associated with an increased LOS in our study. This was inconsistent with previous findings since several other groups have reported higher rates of postoperative complications, postoperative infections and consequently a longer LOS [14, 26-28]. One possible explanation was that comorbidities, which were not examined in our study, could have influenced SMI and SMD values. Few studies have examined the relationship between the presence of comorbidities and muscle abnormalities in the context of cancer. Lieffers et al. in their cohort of 234 CRC patients found a higher prevalence of cardiac arrhythmias, chronic obstructive pulmonary disease, diabetes and other disorders among individuals with low SMI [28]. Xiao et al. demonstrated using a cohort of 3051 patients with stage I to III CRC that pre-existing comorbidities including myocardial infarction, congestive heart failure, peripheral vascular disease, diabetes and renal disease were associated with low SMD [29]. Our observation of equivocal hospitalisation outcomes among patients with normal and abnormal body composition may suggest that our patient cohort had better premorbid health compared to other study populations and therefore have better surgical outcomes in general. However, this speculation needs to be further investigated to be validated.
Finally, our study showed that preoperative inflammation was associated with a longer postoperative LOS. This is consistent with previous studies, which showed that elevated NLR predicted perioperative complications and was associated with increased LOS for patients undergoing resection for CRC [15, 30]. This finding has profound implications for patient care and highlights the usefulness of a simple, inexpensive biomarker that correlates the inflammatory state with colon cancer to its impact on hospitalisation. Preoperative NLR values may be useful for surgeons in terms of identifying high-risk patients postoperatively and allow for early intervention and monitoring of complications.
The prevalence of muscle abnormalities and systemic inflammation in colon cancer patients and their associations with a poorer outcome may serve as a framework for future research to test the hypothesis that interventions which aim to prevent or restrict muscle depletion and dampen inflammation may offer clinical benefit in this population. Although our study did not demonstrate any effect of sarcopenia and myosteatosis on LOS, they are still important factors to consider in cancer patients. These body composition measures may help identify patients with a potentially low tolerance for physical activity or poor diet, which have implications for both short-term and long-term consequences. Using clinically acquired CT images to aid in the early identification of sarcopenia and myosteatosis and serum derived NLR values to measure the systemic inflammatory response could facilitate prehabilitation programmes or therapeutic interventions to promote better outcomes. Treatments might include anti-inflammatory drugs and resistance training programmes which can maintain or increase muscle mass and function in patients with cancer and also improve the quality of life for these patients [31-33]. Future therapeutics to modify muscle abnormalities and reduce inflammation could potentially become an important part of cancer management.
Several limitations need to be noted in our study. Firstly, this study was a single centre retrospective study using data from stored CT scans and serum samples. As we only included patients with an electronically available CT scan, this resulted in a small sample size. Secondly, our study was observational. As in all observational studies, residual confounding is possible. For example, physical activity and diet were not well measured in the electronic medical record and could potentially influence body composition measures. Furthermore, socioeconomic status, alcohol consumption and smoking status were not captured and could plausibly influence muscle mass and quality as well as the inflammatory state. Lastly, we also did not include information regarding comorbidities and surgical techniques, which could influence LOS.
Conclusion
There is increasing interest regarding the underlying pathophysiology of cancer-associated sarcopenia and myosteatosis as well as the role of systemic inflammation and their impact on oncologic outcomes. Our work demonstrated that sarcopenia and myosteatosis were prevalent in colon cancer patients, but there seemed to be no correlation between these two types of muscle abnormalities. We also demonstrated that preoperative systemic inflammation was associated with prolonged hospitalisation. Our work contributes to the growing body of evidence linking changes in body composition and systemic inflammation with clinical outcomes in cancer. These markers are readily available and can become important indicators to identify at-risk phenotypes for consideration of therapeutic interventions.
Abbreviations
BMI body mass index CRC colorectal cancer CT computed tomography HU Hounsfield unit L3 third lumbar LOS length of stay NLR neutrophil-to-lymphocyte ratio OR odds ratio SMD skeletal muscle radiodensity SMI skeletal muscle index SVHM St Vincent’s Hospital Melbourne
Acknowledgement
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 04, Jul 2019Accepted: Fri 26, Jul 2019
Published: Fri 23, Aug 2019
Copyright
© 2023 Wen Hsin. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.ACO.2019.03.01
Author Info
Wei Hong Hannah Rouse Melissa Moore Wen Hsin
Corresponding Author
Wen HsinUniversity of Melbourne, Melbourne, Australia
Figures & Tables
Table 1: Baseline characteristics of the study population.
|
Characteristic |
n = 133 |
|
Age (years) |
69.1 ± 13.8 |
|
Sex |
|
|
Male |
75 (56.4%) |
|
Female |
58 (43.6%) |
|
Cancer stage |
|
|
I |
35 (26.3%) |
|
II |
52 (39.1%) |
|
III |
46 (34.6%) |
|
BMI category |
|
|
Underweight |
4 (3.0%) |
|
Normal |
48 (36.1%) |
|
Overweight |
38 (28.6%) |
|
Obese |
43 (32.3%) |
|
Sarcopenia status |
|
|
Yes |
55 (41.4%) |
|
No |
78 (58.6%) |
|
Myosteatosis status |
|
|
Yes |
52 (39.1%) |
|
No |
81 (60.9%) |
|
Body composition measures |
|
|
BMI (kg/m2) |
26.6 (23.2 - 31.2) |
|
SMI (cm2/m2) |
48.0 (43.0 - 54.8) |
|
SMD (HU) |
38.3 (29.9 - 47.9) |
|
Inflammation status |
|
|
NLR ≥ 3 |
52 (39.1%) |
|
NLR < 3 |
81 (60.9%) |
|
Inflammation measure |
|
|
NLR |
2.6 (1.9 - 4.1) |
|
LOS (days) |
7 (5 - 12) |
Table 2: The relationship between patient characteristics, cancer stage, BMI, NLR and measures of body composition.
|
|
Variable |
Coefficient |
95% CI |
P-value |
|
SMI |
|
|
|
|
|
|
Age |
–0.05 |
-0.13 - 0.03 |
0.181 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
7.39 |
5.18 - 9.60 |
< 0.001 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
0.50 |
–2.32 - 3.33 |
0.725 |
|
|
III |
1.20 |
–1.61 - 4.02 |
0.399 |
|
|
BMI |
0.64 |
0.47 - 0.81 |
< 0.001 |
|
|
NLR |
–0.50 |
–1.08 - 0.08 |
0.089 |
|
SMD |
|
|
|
|
|
|
Age |
–0.20 |
–0.41 - 0.01 |
0.057 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
6.92 |
1.13 - 12.72 |
0.020 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
5.91 |
–1.50 - 13.31 |
0.117 |
|
|
III |
1.01 |
–6.36 - 8.38 |
0.787 |
|
|
BMI |
–0.88 |
–1.32 - –0.43 |
< 0.001 |
|
|
NLR |
1.52 |
–0.00 - 3.03 |
0.050 |
Multivariate linear regression analyses for body composition measures. Abbreviations: BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity; CI, confidence interval.
Table 3: The relationship between patient characteristics, cancer stage, BMI, measures of body composition, NLR and LOS.
|
|
Variable |
OR |
95% CI |
P-value |
|
|
Age |
1.03 |
1.00 - 1.07 |
0.022 |
|
|
Sex |
|
|
|
|
|
Female |
(baseline) |
|
|
|
|
Male |
0.85 |
0.36 - 2.03 |
0.714 |
|
|
Stage |
|
|
|
|
|
I |
(baseline) |
|
|
|
|
II |
1.27 |
0.49 - 3.30 |
0.627 |
|
|
III |
0.86 |
0.33 - 2.22 |
0.751 |
|
|
BMI |
1.00 |
0.93 - 1.08 |
0.929 |
|
|
SMI |
1.00 |
0.94 - 1.06 |
0.994 |
|
|
SMD |
1.00 |
0.98 - 1.03 |
0.800 |
|
|
NLR |
1.29 |
1.04 - 1.61 |
0.019 |
Multivariate logistic regression analysis for LOS. Abbreviations: BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio; LOS, length of stay; SMI, skeletal muscle index; SMD, skeletal muscle radiodensity; OR, odds ratio; CI, confidence interval.



References
- Bowel cancer statistics (2019) New South Wales: Cancer Australia.
- Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57: 58-67. [Crossref]
- Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P et al. (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106: dju124. [Crossref]
- Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT et al. (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31: 1539-1547. [Crossref]
- Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J et al. (2017) Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study). Cancer Epidemiol Biomarkers Prev 26: 1008-1015. [Crossref]
- Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J et al. (2018) Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 124: 3008-3015. [Crossref]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB et al. (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755-763. [Crossref]
- Baracos V, Kazemi-Bajestani SM (2013) Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int J Biochem Cell Biol 45: 2302-2308. [Crossref]
- Sun G, Li Y, Peng Y, Lu D, Zhang F et al. (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33: 1419-1427. [Crossref]
- Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S et al. (2016) Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 5: 607-616. [Crossref]
- Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S et al. (2017) Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: the C-SCANS study. Cancer 123: 4868-4877. [Crossref]
- Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE et al. (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13: 3264-3268. [Crossref]
- Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT et al. (2017) Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 3: e172319. [Crossref]
- Boer BC, de Graaff F, Brusse-Keizer M, Bouman DE, Slump CH et al. (2016) Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis 31 :1117-1124. [Crossref]
- Josse JM, Cleghorn MC, Ramji KM, Jiang H, Elnahas A et al. (2016) The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Colorectal Dis 18: O236-O242. [Crossref]
- Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM et al. (2017) The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol 115: 470-479. [Crossref]
- Shen W, Punyanitya M, Wang Z, Gallagher D, St.-Onge MP et al. (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97: 2333-2338. [Crossref]
- Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ et al. (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33: 997-1006. [Crossref]
- Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST et al. (2019) The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 10: 111-122. [Crossref]
- Baracos VE, Arribas L (2018) Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncolo 29: ii1-ii9. [Crossref]
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB et al. (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9: 629-635. [Crossref]
- Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Kroenke CH et al. (2019) The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am J Clin Nutr 109: 615-625. [Crossref]
- Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH et al. (2016) Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 263: 320-325. [Crossref]
- Richards CH, Roxburgh CS, MacMillan MT, Isswiasi S, Robertson EG et al. (2012) The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS ONE 7: e41883. [Crossref]
- McSorley ST, Black DH, Horgan PG, McMillan DC (2018) The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr 37: 1279-1285. [Crossref]
- Martin L, Hopkins J, Malietzis G, Jenkins JT, Sawyer MB et al. (2018) Assessment of computed tomography (CT)-defined muscle and adipose tissue features in relation to short-term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol 25: 2669-2680. [Crossref]
- van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT et al. (2018) Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol 44: 1354-1360. [Crossref]
- Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE (2012) Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 107: 931-936. [Crossref]
- Xiao J, Caan BJ, Weltzien E, Cespedes Feliciano EM, Kroenke CH et al. (2018) Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J Cachexia Sarcopenia Muscle 9: 654-663. [Crossref]
- Gohil R, Rishi M, Tan BH (2014) Pre-operative serum albumin and neutrophil-lymphocyte ratio are associated with prolonged hospital stay following colorectal cancer surgery. Br J Med Med Res 4: 481-487. [Crossref]
- Ewaschuk JB, Almasud A, Mazurak VC (2014) Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab 39: 654-662. [Crossref]
- Focht BC, Clinton SK, Devor ST, Garver MJ, Lucas AR et al. (2013) Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol 11: 45-60. [Crossref]
- Padilha CS, Marinello PC, Galvao DA, Newton RU, Borges FH et al. (2017) Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv 11: 339-349. [Crossref]