Semi-Percutaneous Short-Term Support of Right Heart Failure in Acute Aortic Dissection
A B S T R A C T
Coronary malperfusion in acute aortic dissection type A is associated with increased mortality. The present case shows the management of a patient with an acute right ventricular infarction due to coronary avulsion, with the coronary bypass performed first, followed by aortic surgery and temporary extracorporeal right ventricular support, which was successfully weaned percutaneously after recovery while maintaining a low anticoagulation strategy.
Keywords
Dissection type A, coronary malperfusion, right heart infarction, temporary extracorporeal right ventricular support
Introduction
Acute aortic dissection type A (AADA) is associated with an in-hospital mortality of about 18% among patients assigned to emergency surgical care, and preoperative coronary mal-perfusion is one independent predictor [1, 2].
Patient and Methods
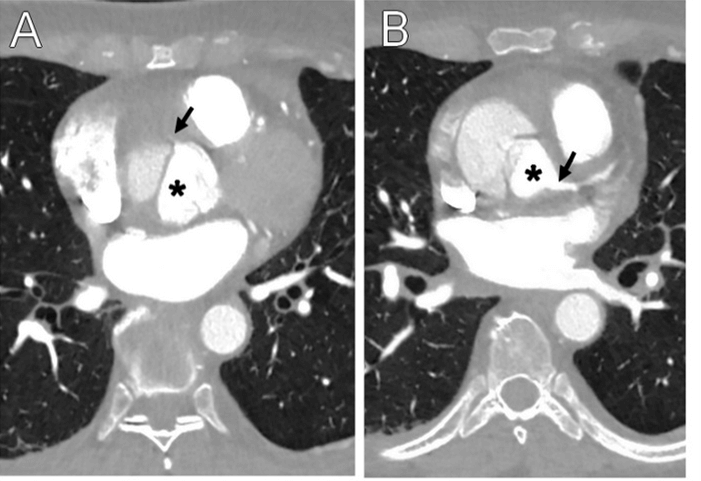
A 55-year-old woman was admitted with chest pain. The ECG showed non-specific ST depression. CT scan showed AADA (Figure 1). Echocardiography revealed moderate right ventricular (RV) dysfunction and aortic valve insufficiency. After median sternotomy, heparinization, innominate artery and central venous cannulation, cardiopulmonary bypass (CPB) was started. After cross-clamping and aortic incision, cardioplegia was injected directly into the common trunk (using a flexible cannula with a soft tip) with rapid cardiac arrest. Aortic regurgitation through a bicuspid valve was due to a dilated aortic annulus, and the intimal tear was located in the ascending aorta. During the subsequent cardioplegia injection into the ostium of the right coronary artery (RCA) a reflux through the false lumen of the residual proximal aorta was observed. The preparation of the ostium showed a complete circumferential avulsion, i.e., the separation of all coronary wall layers, and a retraction of the remaining RCA. A saphenous vein was quickly harvested and anastomosed to the RCA, and the cardioplegic protection was then completed by injection via the inverted graft.
Figure 1: Dissection at the coronary plane shows A) A strongly reduced perfusion of right coronary artery (arrow) and B) A normal via the true lumen (asterisk) perfused left coronary artery (arrow).
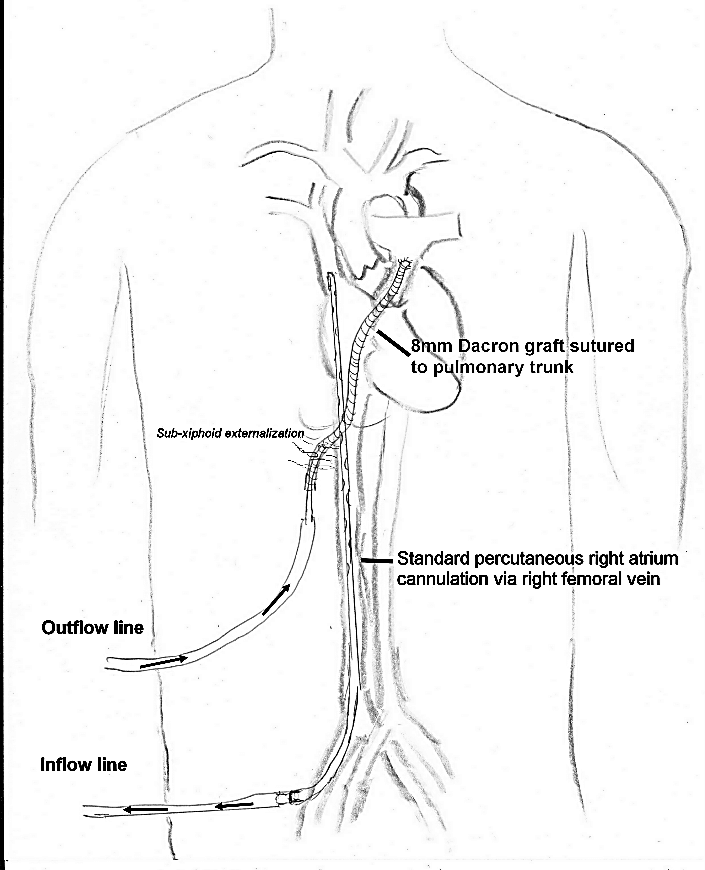
A composite Bentall procedure (neo-sinus Dacron tube with inserted aortic valve bio-prosthesis), with subsequent hemi-arch replacement, under hypothermic circulatory arrest (28°) and selective antegrade cerebral perfusion via the TABC (with clamped left common carotid artery), was performed, at the end of which the venous bypass was anastomosed to the prosthetic neo-aorta. After cross-clamp removal, rewarming, and recovery of sinus rhythm, an attempt was made to wean CPB. The echo showed severe RV dysfunction with normal left ventricular function, and it was decided to install a temporary extracorporeal right ventricular assistance (eR-VAD). The right atrium was cannulated via the femoral vein (Maquet HLS Bioline 23 Fr / 55 cm), and an 8 mm Dacron tube was sutured to the side-clamped pulmonary trunk, passed through the retrosternal space, and externalized in the sub-xiphoid region. The femoral cannula was connected to the “venous” inflow line and a standard arterial cannula (Maquet HLS Bioline 17 Fr / 23 cm) was inserted and secured in the Dacron graft and connected to the “arterial” outflow line of a circuit with a centrifugal pump (Levitronix CentriMag®) (Figure 2). The sternum was closed after CPB weaning, and the patient was transferred to the intensive care unit.
Figure 2: Schematic illustration of the semi-percutaneous extracorporeal right ventricular support.
Prophylactic antithrombotic therapy with unfractionated heparin was started 6 h later and maintained during assistance. RV function improved (also as a result of a continuous 24-hour injection of levosimendan), and the eR-VAD was successfully weaned on day 10. The cannulas were removed percutaneously at the patient's bedside, i.e., the Dacron tube was cut and overstitched under slight tension near the skin, allowing its free end to retract. At the 2-month follow-up, a slight to moderate RV dysfunction was documented.
Discussion
According to the GERAADA registry, coronary malperfusion is an independent predictor of hospital mortality [2]. Preoperative detection of coronary malperfusion can be difficult and maybe resolved by aortic root surgery that restores physiological blood flow. A complete coronary avulsion is rare but requires rapid revascularization. The use of an isolated rotary pump (rather than an ECMO) has the advantage of avoiding complete anticoagulation, and the semi-percutaneous approach allows eR-VAD ablation without reopening the sternum. Luciani et al. have described in 1996 a successful temporary RV assistance in the context of an infraction in AADA using a Bio-Medicus centrifugal pump. The RCA was stenosed (and not occluded) due to the false lumen protrusion [3]. In another case of RV infarction, initially treated by root replacement and direct RCA re-implantation, coronary occlusion occurred in the postoperative course, which finally required a change from temporary to long-term assistance [4]. Mashar et al. reported a case of RV ischaemia due to technical problems of the RCA anastomosis requiring emergency venous bypass and temporary assistance, using the Levitronix Centri Mag® [5]. However, the anatomical conditions causing ischaemia varies, and the authors do not discuss their anticoagulation management nor any benefits depending on the configuration for RV support.
Conclusion
The case points to the importance of immediate revascularization of coronary occlusion in the context of AADA, as well as to the technical aspects of a semi-percutaneous eR-VAD, which allows bedside weaning.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Tue 16, Mar 2021Accepted: Wed 31, Mar 2021
Published: Fri 16, Apr 2021
Copyright
© 2023 Lars Niclauss. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.04.07
Author Info
Lars Niclauss Aurelien Roumy Matthias Kirsch
Corresponding Author
Lars NiclaussDepartment of Cardiovascular Surgery, Lausanne University Hospital CHUV, Lausanne, Switzerland
Figures & Tables
References
1. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD et al. (2018) Insights from the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 137: 1846-1860. [Crossref]
2. Czerny M, Siepe M, Beyersdorf F, Feisst M, Gabel M et al. (2020) Prediction of mortality rate in acute type A dissection: the German Registry for Acute Type A Aortic Dissection score. Eur J Cardiothorac Surg 58: 700-706. [Crossref]
3. Luciani GB, Fabbri A, Faggian G, Marino P, Mazzucco A (1993) Mechanical assist device for right ventricular failure after acute aortic dissection. Ann Thorac Surg 56: 363-366. [Crossref]
4. Marquez TT, D'Cunha J, John R, Liao K, Joyce L (2009) Mechanical support for acute right ventricular failure: evolving surgical paradigms. J Thorac Cardiovasc Surg 137: e39-e40. [Crossref]
5. Mashar RP, Birla RP, Waterworth PD (2019) Right Ventricular Failure Following Acute Type A Aortic Dissection: An Alternative Strategy. World J Cardiov Surg 9: 35 -39.
