Journals
Safety and efficacy of bone marrow mononuclear cell transplantation in the treatment of decompensated liver cirrhosis
A B S T R A C T
Aims: To evaluate the safety and efficacy of autologous bone marrow mononuclear cells (BM-MNCs) in the treatment of patients with decompensated liver cirrhosis.
Methods: We randomly assigned 34 patients to control (18 cases) and BM-MNCs (16 cases) groups. All patients in the BM-MNCs group received granulocyte colony-stimulating factor for 3 days and were then infused with BM-MNCs. We then tested liver function, blood coagulation parameters, Karnofsky performance status (KPS), and the levels of procollagen IIIN peptide and hyaluronic acid.
Results: Generally, the symptoms of patients in the BM-MNCs group improved significantly compared to controls. Six months after transplantation, total bilirubin level was 14.94±7.20μmol/L vs 24.50±15.34μmol/L (p=0.045), albumin level was 40.65±4.94g/L vs 34.78±4.92g/L (p=0.000), and prothrombin time was 10.86±1.18s vs 13.49±1.80s (p=0.002), respectively. KPS significantly improved when measured one month and six months after BM-MNCs therapy and showed obvious differences compared to controls. Changes in Child-Pugh score were also statistically significant when measured six months after therapy (p=0.001). Mean pretreatment serum baseline levels of procollagen IIIN peptide and hyaluronic acid were significantly lower than those measured one month after therapy (both p < 0.05).
Conclusions: Autologous transplantation of BM-MNCs is safe and effective for patients with decompensated liver cirrhosis and leads to improvements in liver function, hepatic Child-Pugh score and KPS.
K E Y W O R D S
Bone marrow mononuclear cells, autologous, transplantation, liver cirrhos
I N T R O D U C T I O N
Liver cirrhosis is the final pathological stage of chronic liver disease, is characterized by portal hypertension and functional impairment of the liver and is very difficult to treat [1]. Until now, liver transplantation has been the only way in which clinicians could attempt to cure liver cirrhosis. However, due to the severe shortage of organ donors, surgical complications, immune rejection and high costs, the clinical application of transplantation is extremely limited for patients with decompensated cirrhosis [2, 3]. Therefore, it is critical that we explore new, safe, feasible and effective treatment measures for liver cirrhosis. Over recent years, the mechanisms underlying liver cirrhosis have become widely elucidated. We now know that a variety of cells, cytokines and extracellular matrix are involved in the formation and development of liver cirrhosis [4-8]. Stem cells are a type of self-replicating and differentiating cell with specific functions and have led to significant innovation in regenerative medicine [9]. A range of studies have reported the use of mesenchymal stem cell transplantation for a variety of diseases such as ischemic heart disease and diabetes. While several studies have investigated the effect of hematopoietic stem cells and/or bone marrow mononuclear cells (BM-MNCs) on patients with liver cirrhosis, there has been little research carried out on BM-MNCs. Some reports [10, 11] have indicated that the transplantation of BM-MNCs would be of benefit to patients with decompensated cirrhosis, while others [12] have reported only a transient improvement in the model for end stage liver disease (MELD) score in subjects treated with CD133+ cells, but not in an MNC or placebo group. Furthermore, the effect of BM-MNCs on fibrosis of the liver, and on the quality of life of patients with liver cirrhosis, is still poorly understood.
Therefore, the aim of our study was to ascertain the safety and efficacy of BM- MNCs in the treatment of patients with decompensated liver cirrhosis by comparing data from patients, injected with or without BM-MNCs, relating to liver function, blood coagulation parameters, Karnofsky performance status, Child-Pugh score and biomarkers of fibrosis.
Methods Patients
Between October 2013 and July 2015, a total of 34 patients with decompensated liver cirrhosis were recruited from Qilu Hospital of Shandong University. Then, the patients were randomly allocated into two groups, a control group (18 cases) and an experimental group (16 cases) (Fig. 1). The control group received only standard of care management. All patients provided informed written consent after the benefits, risks, and potential complications of the study had been explained. Patients received BM-MNCs therapy once, in addition to standard of care therapy.
Patients were eligible for the study if they met the following criteria: 1) 18-70 years old with a clinical diagnosis of decompensated liver cirrhosis; 2) Not undergoing liver transplantation within the next six months; 3) Not undergoing any form of treatment except for antiviral drugs; 4) No history of immunosuppressive therapy.
The exclusion criteria were as follows: 1) Pregnancy or lactation; 2) Severe coagulation dysfunction; 3) Experience of gastrointestinal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis or fever of undetermined origin over the last month; 4) Suffering with hepatocellular carcinoma or other malignancies and severe infectious disease; 5) Positive iodine allergy test; 6) Failure or refusal to provide informed signed consent. This study was approved by the Ethics Committee of Qilu Hospital of Shandong University.
Symptoms, signs, auxiliary results and Child-Pugh and KPS scores
Data regarding symptoms and signs, such as lower limb edema, ascites and jaundice, liver function tests, and KPS scores were acquired and recorded. Then, according to the related data, the Child-Pugh score was determined for each patient. All data were analyzed to investigate the effect of BM-MNCs transplantation.
Preparation of bone marrow cells and autologous BM-MNCs transplantation
Patients in the BM-MNCs group received a daily subcutaneous injection of 100 μg granulocyte colony-stimulating factor (G-CSF) for 3 days. Following local anesthesia, 80–100 ml of bone marrow was collected from the bilateral posterior superior iliac spine of each patient using 4000 U of heparin for anticoagulation. BM-MNCs were then isolated by Ficoll-Paque density centrifugation in a laminar air-flow hood. The bone marrow was then centrifuged at 2000 rpm for 20 min to remove upper fat and some plasma, and then diluted with normal saline in a 1:1 ratio. Then, 30 ml of diluted blood cells were gently added to 15 ml of Ficoll separating medium, followed by centrifugation at 1600 rpm for 20 min. Cells from the interphase were obtained and washed three times with 40 mL of normal saline by centrifugation at 1500 rpm for 15 min. Cells were then resuspended in 50 ml of physiological saline (containing 1% human serum albumin), and viability was assessed. The mean number of mononuclear cells in such preparations was 2.13 ± 0.67 × 108 (range: 1.06–3.42× 108) with more than 99% cell viability.
The infusion of bone marrow mononuclear cells was performed on the same day as bone marrow collection in a laminar flow sterile treatment room. The femoral artery was punctured followed by the insertion of a 0.032-inch guide wire and a 4.0F catheter. The celiac trunk artery was then observed under angiography, meanwhile excluding the presence of liver cancer. Then, the common hepatic artery was selected using a 2.1F micro-catheter and 50 ml of isolated cell suspension was slowly injected using digital subtraction angiography; following injection, the catheter was rinsed with 20 ml of normal saline.
Levels of procollagen IIIN peptide (PIIINP) and hyaluronic acid (HA)
A 5-ml fasting blood sample was collected form each patient pre-transplantation and one month postoperatively. These samples were centrifuged at 3000 rpm for 20 min at 4°C and serum samples were stored at -70°C to await further analysis. The fibrosis biomarkers, PIIINP and HA, were then evaluated via enzyme-linked immune-sorbent assay (ELISA).
Follow-up
One and six months after the procedure, patients returned to the outpatient department for clinical and laboratory evaluation including symptoms and signs, liver function tests, coagulation parameters, PIIINP and HA levels, and Child-Pugh and KPS scores.
Statistical analysis
All data were analyzed by SPSS 19 software and P < 0.05 was considered to be statistically significant. Data from clinical and biochemical analyses were expressed as mean ± standard deviation (SD) and compared using χ 2 and t tests.
Tables
Figures
Figure 1: Flow chart indicating the selection process for this study.
Results
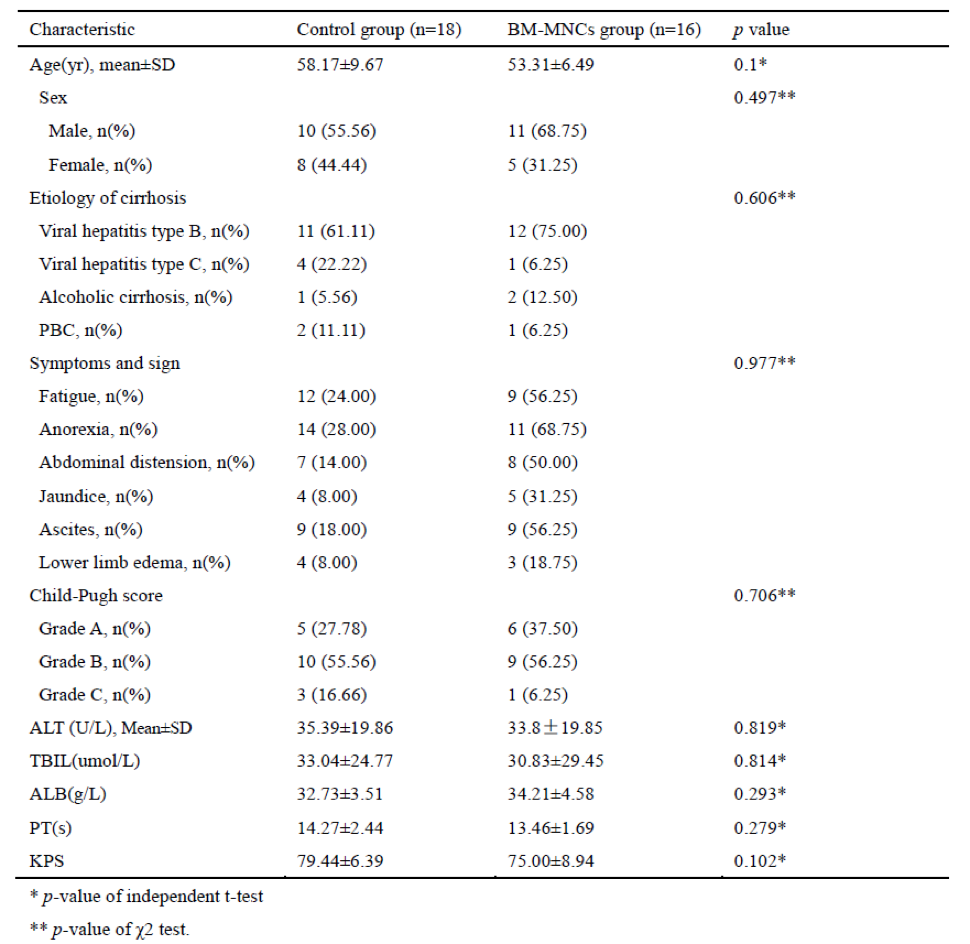
A total of 34 patients (21 men; 61.76%) were recruited into the study. In all, the mean age at recruitment was 55 years (range, 42–84 years old). There was no significant difference in terms of either mean age or gender between the control and BM-MNCs groups. The baseline etiology of cirrhosis, symptoms and signs, glutamic-pyruvic transaminase (ALT), total bilirubin (TBIL), albumin (ALB), prothrombin time (PT) levels, Karnofsky performance status (KPS) and Child-Pugh scores did not differ significantly between the two groups. The baseline characteristics of the patients are summarized in Table 1.
Six months after transplantation, two patients in the control group with a Child-Pugh C score died of upper gastrointestinal hemorrhage. In the BM-MNC group, two patients with alcoholic cirrhosis and PBC died of upper gastrointestinal hemorrhage and liver failure, respectively; one additional patient was lost to follow-up.
Symptoms, syndromes and complications
Over the six-month follow-up period, most of the patients showed satisfactory improvement, but with differing degrees of symptoms and syndromes; there was no significant difference in this respect when compared between the two groups.
The incidence of complications was monitored throughout the six-month follow-up period. A total of 10 patients in the two groups were observed with postoperative fever, with temperatures ranging from 37.4°C to 38.6°C; fever generally lasted 2–3 days. No serious complications were observed during the follow-up period, and there was no evidence of neoplastic disease.
Liver function tests and KPS scores
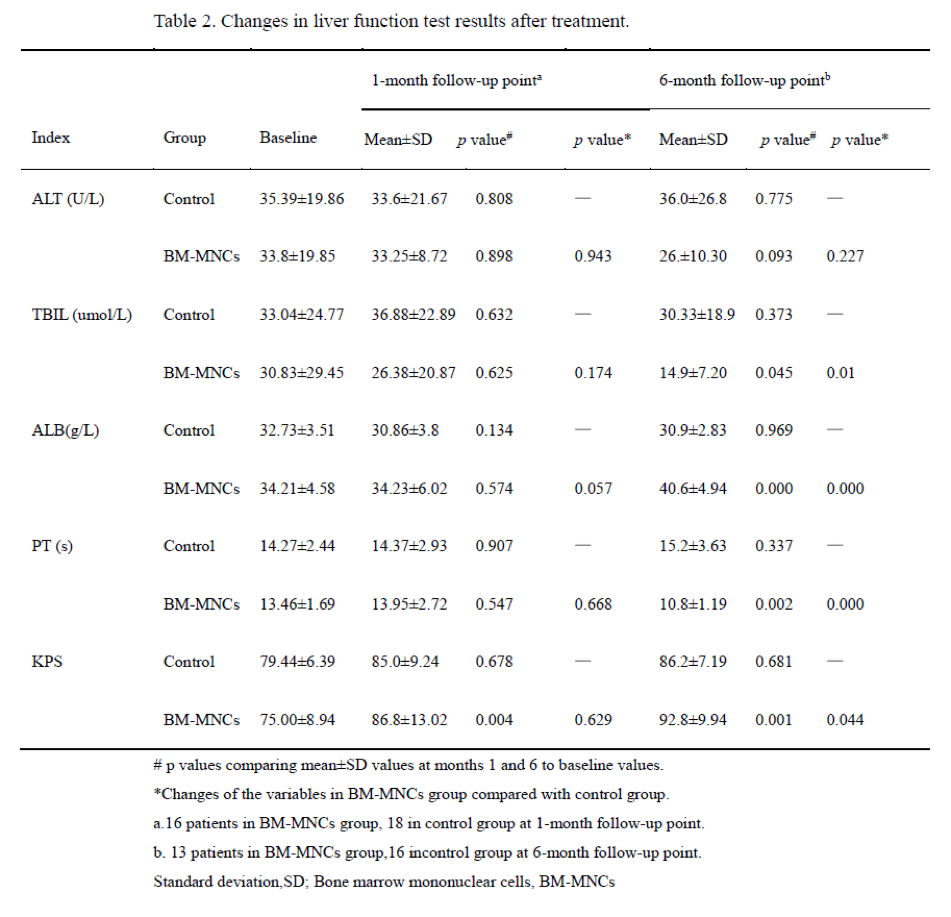
During the one-month follow-up period, glutamic-pyruvic transaminase (ALT), total bilirubin (TBIL), albumin (ALB), prothrombin time (PT) levels, and Karnofsky performance status (KPS) did not differ significantly between the two groups. However, KPS scores improved significantly in the BM-MNCs group compared with scores prior to transplantation (P=0.004). After six months, the TBIL, ALB, PT levels and KPS scores in the BM-MNCs group had improved significantly compared with values prior to transplantation (P=0.045, P=0.000, P=0.002 and P=0.001, respectively); these scores were also significantly different when compared to the control group (P < 0.05; Table 2).
Child-Pugh Score
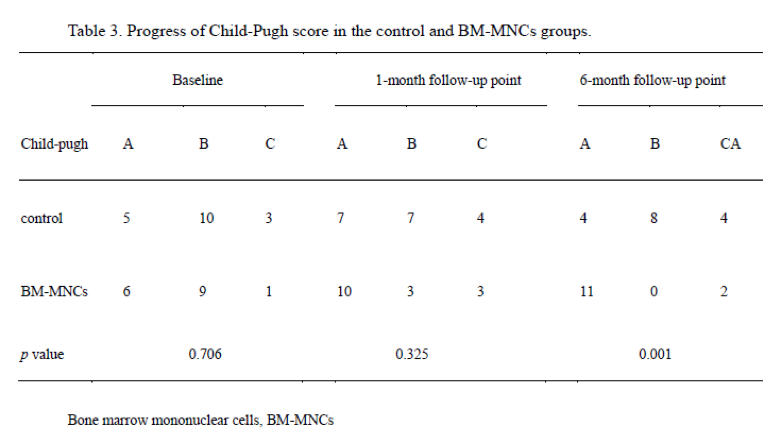
In the BM-MNCs group, the change in the number of patients with Child-Pugh A, Child-Pugh B and Child-Pugh C scores before transplantation when compared with those one month later were 6 vs 10, 9 vs 3, and 1 vs 3, respectively. There was no significant difference between the two groups. At six months, the number of patients with Child-Pugh A, Child-Pugh B and Child-Pugh C scores was 11, 0 and 2, respectively in the BM-MNCs group, showing a significant improvement compared with the control group (p=0.001; Table 3).
Levels of PIIINP and HA
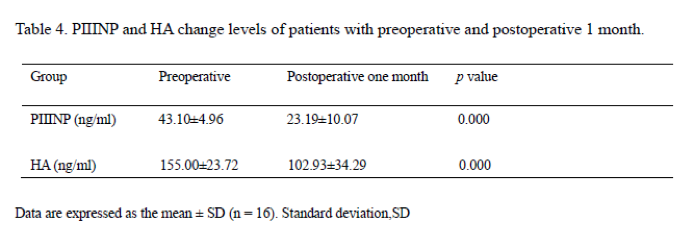
One month after transplantation, the levels of PIIINP and HA had reduced significantly compared with pre-transplantation levels (PIIINP: 43.10±4.96 ng/ml to 23.19±10.07ng/ml; HA: 155.00±23.72ng/ml to 102.93±34.29ng/ml) (P=0.000; Table 4).
Discussion
In this study, we infused BM-MNCs via the proper hepatic artery into patients with decompensated liver cirrhosis; this proved to be a feasible and safe procedure. Furthermore, the symptoms of patients in the BM-MNCs group improved significantly compared to controls
BM-MNCs therapy has the capability for self-renewal and pluripotency, and may inhibit the activation of hepatic stellate cells (HSCs), reduce the expression of collagen I and oxidative stress, protect mitochondrial function, down-regulate pro-inflammatory cytokines, promote liver regeneration and alleviate hepatic fibrosis [13]. In a previous study, Bai et al. [11] reported that the liver function parameters, coagulation function and routine blood tests of 32 patients with decompensated liver cirrhosis were significantly improved just one month after the transfusion of BM-MNCs. Similarly, our present results also showed that after six months, serum albumin levels after injection were obviously elevated, and that total bilirubin and PT levels had been significantly reduced in the BM-MNCs group. In addition, PIIINP [14] and HA [15] are two important serological markers of hepatic fibrosis or cirrhosis; these were also significantly reduced just one month after the transfusion of BM-MNCs.
However, the mechanism of how BM-MNCs are able to inhibit fibrosis remains unclear. A preclinical study showed that the infusion of bone-marrow-derived cultured cells could significantly improve serum albumin, liver fibrosis and liver function in mice with CCl4-induced cirrhosis [16]. Tanimoto et al. [17] found that the increased level of matrix metalloproteinase (MMP)-9, and suppressed activation of HSCs, occurring in response to regulation by humoral factors (TNFα and TGFβ), contributed to the improvement of liver fibrosis by mesenchymal stem cells (MSCs) in the mouse carbon tetrachloride (CCl4) liver cirrhosis model. The precise mechanism underlying the inhibitory effect of BM-MNCs upon fibrosis in humans thus warrants further studies.
The Karnofsky Performance Status (KPS) was developed by Karnofsky et al. in 1948 and is a simple, 11-point scale, expressed as a percentage of physical function ranging from 100% (normal, no complaints, no evidence of disease) to 0% (dead) [18, 19]. The KPS has been widely used to stratify the functional status of patients with malignancy to help guide treatment and clinical trials. Over the past two decades, the KPS has been widely indicated to be a reliable and valuable screening modality that can be readily administered in any clinical setting [20, 21].
Recently, Orman et al. [19] performed a retrospective cohort study to examine the KPS scale in patients with cirrhosis listed for liver transplantation and found that KPS was directly and significantly associated with mortality. We also utilized the KPS scale in the present study to evaluate the functional status of patients after the injection of BMCs and found that KPS scores were significantly improved at both one and six months after transplantation, as well as compared with the control group.
Unfortunately, only a very small number of studies have attempted to investigate the effect of autologous BM-MNCs transplantation for cirrhosis arising from different causes. Some studies [22, 23] have shown that autologous BM-MNCs transplantation can significantly improve liver function and the general situation of patients with viral hepatitis cirrhosis in just six months; whereas other reports [24, 25] regarding autologous BM-MNCs transplantation for the treatment of alcoholic cirrhosis, were without a unified opinion. The results of our present study, which involved the treatment of patients with viral hepatitis cirrhosis, are consistent with earlier reports in that liver function, and related detection indices, of patients with viral hepatitis and cirrhosis were significantly improved following the infusion of BM-MNCs. However, only two patients with alcoholic cirrhosis were included in our present study. One of these patients showed an improvement in ascites and bilirubin just one month after BM-MNCs transplantation, and Child-Pugh grade improved from grade B to grade A. Unfortunately, the condition of the other case did not improve; Child-Pugh grades were Grade C at both the one- and six-month follow-up. As our study only included two cases with alcoholic cirrhosis in the BM-MNCs group, it was not possible to draw any conclusion with regards to this condition; further studies are required.
There were some limitations in our study. First, our study did not include pathological histology because patients refused to undergo biopsy. Second, the follow-up time was short.
In conclusion, autologous BM-MNCs transplantation is safe and effective for patients with decompensated liver cirrhosis and leads to improvements in liver function, hepatic Child-Pugh score and Karnofsky performance status. In future, BM-MNCs transplantation is expected to become a new therapy for the treatment of liver cirrhosis.
Acknowledgements
The authors would like to thank Qilu Hospital of Shandong University (China).
Conflict of Interest
There are no conflicts of interest.
Article Info
Article Type
Research ArticlePublication history
Received: Sun 20, May 2018Accepted: Wed 30, May 2018
Published: Mon 11, Jun 2018
Copyright
© 2023 Yangjing Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2018.02.002
Author Info
Baoquan Cheng Tao Zhou Xianghua Cui Yangjing Gao Zhen Zhou Zhi Peng
Corresponding Author
Yangjing GaoDepartment of Gastroenterology, Qilu Hospital of Shandong University, Jinan, China
Figures & Tables





References
1. Zhou WC, Zhang QB, Qiao L (2014) Pathogenesis of liver cirrhosis. World J Gastroenterol 20: 7312-24. [Crossref]
2. Wang X, Lin SX, Tao J, Wei XQ, Liu YT, et al. (2014) Study of liver cirrhosis over ten consecutive years in Southern China. World J Gastroenterol 20: 13546-13555. [Crossref]
3. Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, et al. (2004) Factors affecting graft survival after living donor liver transplantation. Transplant Proc 36: 2255-2256. [Crossref]
4. Saxena NK, Anania FA (2015) Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab 26: 153-161. [Crossref]
5. Bonefeld K, Moller S (2011) Insulin-like growth factor-I and the liver. Liver Int 31: 911-919. [Crossref]
6. Wilhelm A, Shepherd E L, Amatucci A, Munir M, Reynolds G, Humphreys E, et al. (2016)Interaction of TWEAK with Fn14 leads to the progression of fibrotic liver disease by directly modulating hepatic stellate cell proliferation. J Pathol 239: 109-121. [Crossref]
7. Lee JI, Wright JH, Johnson MM, Bauer RL, Sorg K, et al. (2016) Role of Smad3 in platelet-derived growth factor-C-induced liver fibrosis. Am J Physiol Cell Physiol 310: 436-445. [Crossref]
8. Xu F, Liu C, Zhou D, Zhang L (2016) TGF-beta/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem 64: 157-167. [Crossref]
9. Moore KA, Lemischka IR (2006) Stem cells and their niches. Science 311: 1880-1885. [Crossref]
10. Pankaj P, Zhang Q, Bai XL, Liang TB (2015) Autologous bone marrow transplantation in decompensated liver: Systematic review and meta-analysis. World J Gastroenterol 21: 8697-8710. [Crossref]
11. Bai YQ, Yang YX, Yang YG, Ding SZ, Jin FL, et al. (2014) Outcomes of autologous bone marrow mononuclear cell transplantation in decompensated liver cirrhosis. World J Gastroenterol 20: 8660-8666. [Crossref]
12. Mohamadnejad M, Vosough M, Moossavi S, Nikfam S, Mardpour S, et al. (2016) Intraportal Infusion of Bone Marrow Mononuclear or CD133+ Cells in Patients with Decompensated Cirrhosis: A Double-Blind Randomized Controlled Trial. Stem Cells Transl Med 5: 87-94. [Crossref]
13. Sun CK, Chen CH, Kao YH, Yuen CM, Sheu JJ, et al. (2011) Bone marrow cells reduce fibrogenesis and enhance regeneration in fibrotic rat liver. J Surg Res 169: 15-26.
14. Sajewicz Z, Poniewierka E (1990) Procollagen Type III-N-terminal peptide as an indicator of hepatic fibrogenesis. Pol Tyg Lek 45: 325-326. [Crossref]
15. Neuman MG, Cohen LB, Nanau RM (2016) Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem 49: 302-315. [Crossref]
16. Iwamoto T, Terai S, Hisanaga T, Takami T, Yamamoto N, et al. (2013) Bone-marrow-derived cells cultured in serum-free medium reduce liver fibrosis and improve liver function in carbon-tetrachloride-treated cirrhotic mice. Cell Tissue Res 351: 487-495. [Crossref]
17. Tanimoto H, Terai S, Taro T, Murata Y, Fujisawa K, et al. (2013) Improvement of liver fibrosis by infusion of cultured cells derived from human bone marrow. Cell Tissue Res 354: 717-728. [Crossref]
18. Tandon P, Reddy KR, O'Leary JG, Garcia-Tsao G, Abraldes JG, et al. (2017) A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology 65: 217-224. [Crossref]
19. Orman ES, Ghabril M, Chalasani N (2016) Poor Performance Status Is Associated with Increased Mortality in Patients with Cirrhosis. Clin Gastroenterol Hepatol 14: 1189-1195. [Crossref]
20. Johnson MJ, Bland JM, Davidson PM, Newton PJ, Oxberry SG, et al. (2014) The relationship between two performance scales: New York Heart Association Classification and Karnofsky Performance Status Scale. J Pain Symptom Manage 47: 652-658. [Crossref]
21. Peus D, Newcomb N, Hofer S (2013) Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 13: 72. [Crossref]
22. Kim JK, Park YN, Kim JS, Park MS, Paik YH, et al. (2010) Autologous bone marrow infusion activates the progenitor cell compartment in patients with advanced liver cirrhosis. Cell Transplant 19: 1237-1246. [Crossref]
23. Lukashyk SP, Tsyrkunov VM, Isaykina YI, Romanova ON, Shymanskiy AT, et al. (2014) Mesenchymal Bone Marrow-derived Stem Cells Transplantation in Patients with HCV Related Liver Cirrhosis. J Clin Transl Hepatol 2: 217-221. [Crossref]
24. Spahr L, Chalandon Y, Terraz S, Kindler V, Rubbia-Brandt L, et al. (2013) Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One 8: 53719. [Crossref]
25. Saito T, Okumoto K, Haga H, Nishise Y, Ishii R, et al. (2011) Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev 20: 1503-1510. [Crossref]
