Role of Wnt Signaling Pathways in Clear Cell Renal Cell Carcinoma Pathogenesis in Relation to VHL and HIF Status
A B S T R A C T
Renal cell carcinoma (RCC) encompasses various tumor types characterized by a variety of genetic abnormalities. The genetic changes, like mutations, deletions, and epigenetic alterations, can affect the signaling components and signaling networks, causing the modification of tumor pathogenesis and prognosis of RCC. The most prevalent RCC, clear cell RCC (ccRCC), is asymptomatic in the early stages, refractory to chemotherapy and radiation therapy, and has a poorer prognosis compared with the papillary and chromophobe ccRCC types. Loss of the VHL gene and upregulation of oxygen sensors, hypoxia-inducible factor alphas (HIF-α), which promote different growth factors, is a signature of sporadic ccRCC. The VHL-HIF-α and Wnt/β-catenin pathways are closely connected and contribute to the ontogeny of ccRCC. This review confines to ccRCC and the role of the Wnt/β-catenin signaling pathways and its crosstalk with VHL/HIF.
Keywords
ccRCC, VHL/HIF, Wnt/β-catenin signaling pathway
Introduction
Renal cell carcinoma (RCC) is composed of a heterogeneous group of tumors originating from different parts of the nephron, maintaining distinct genetic and histological characteristics, which is more prevalent in men (5%) than in women (3%) worldwide [1]. The most commonly occurring RCC types are clear cell (ccRCC), papillary (pRCC), and chromophobe RCC (chRCC) [2]. The clear cell type represents 70–80% of RCC, and the 5-year overall survival rate is about 55-60%, whereas the non-ccRCC types, pRCC and chRCC, constitute about 15% and 10% of RCC, respectively with 5-year survival rates around 70–95% [3]. The non-ccRCC types show the aberrations of discrete genes and signaling pathways and display a hypovascular feature [4, 5]. Hence, non-ccRCC tumors differ in tumor progression and tumor behavior compared with ccRCC. The most common genetic aberrations encountered in ccRCCs are loss chromosome 3p25, leading to the inactivation of the von Hippel–Lindau (VHL) gene [6]. In sporadic ccRCC, loss of heterozygosity (LOH), mutation and deletion, or hypermethylation of the VHL gene demonstrate gene inactivation in 56%-91% of the cases [7-11]. In sporadic ccRCC, lack of pVHL activated the transcription factor hypoxia-inducible factor-alpha (HIF-) subunits and further modifies several growth factors, like epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) [12]. In ccRCC, VHL not only regulates the cellular oxygen sensing mechanism but is also involved in Wnt/β-catenin mediated signaling pathways [13, 14].
The Wnt signaling pathway is classified into the canonical or β-catenin-dependent or Wnt/β-catenin and non-canonical or β-catenin-independent pathway. The non-canonical pathway, which is not discussed further in this review, is linked with cytoskeleton remodeling and cell movement. The canonical signaling pathway is crucial for normal embryonic development and cell activities, such as cell proliferation and stem cell renewal. Also, dysregulation of the pathway causes carcinogenesis and metastatic behavior [15, 16]. The canonical pathway propagates its functions through the overexpression of β-catenin. Several factors, at different layers of the Wnt signaling pathway, can cause the upregulation of β-catenin such as mutations in the β-catenin gene, and mutations in axin and adenomatous polyposis coli (APC), dysregulation of the β-catenin destruction complex, overexpression of Wnt ligands, downregulation of Wnt antagonists and loss of inhibition or decreased activity of regulatory pathways [15-17].
This review summarizes the canonical Wnt/β-catenin signaling pathway and its role in the oncogenesis of ccRCC in the presence or absence of VHL gene function.
The Canonical Wnt Signaling Pathway (Wnt/ β-catenin)
Generally, the canonical pathway is constituted of the following factors: the ligand/receptor cell membrane complex, the cytosolic β-catenin destruction complex, and the nuclear β-catenin- T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription complex [18, 19].
Figure 1: Schematic diagram of the Wnt/b-catenin signaling pathway in general and ccRCC.
A) Wnt-OFF. When Fzd/LRP receptors are not engaged, the destruction complex [in the dotted circle: CK-1, GSK-3β, APC, and Axin-1] phosphorylates β-catenin at various residues and destabilizes β-catenin. Consequently, β-catenin undergoes proteasomal degradation.
B) Wnt-ON. Binding of Wnt ligands trigger membrane receptors Fzd /LRP and deactivate the destruction complex; as a result, stabilized β-catenin accumulate in the cytoplasm and then translocated into the nucleus to enhance the transcription of the Wnt target genes with co-factor TCF/LEF.
C) Wnt-in ccRCC. In ccRCC, secreted Wnt ligands bind to Wnt membrane receptors, or loss of function/ deletion/ promotor hypermethylation of Wnt antagonists or elevated expression of Fzds disrupt the destruction complex (in the dotted circle). Subsequently, non-phosphorylated β-catenin translocate into the nucleus, and in association with TCF/LEF stimulate Wnt/ β-catenin pathway and further activate Wnt target genes like cMYC, cyclin D1; Loss of pVHL in ccRCC activates Wnt/ β-catenin pathway not only by stabilizing HIF-1α but also by destabilizing JADE1.
In normal conditions, in the absence of Wnt ligand, the Wnt proteins form the “destruction complex” that includes the tumor suppressors APC, glycogen synthase kinase 3β (GSK-3β), casein kinase 1 (CK1), protein phosphatase 2A, and ubiquitin-labeled and the E3-ubiquitin ligase Beta-transducin repeats-containing proteins (β-TrCP). The destruction complex, by series of phosphorylation events, maintains a low level of β-catenin in the cytoplasm and as a result, the Ubiquitin-Proteasome System (UPS) degrades phosphorylated β-catenin (Figure 1A) [17, 20-24].
Induction of the canonical Wnt signaling pathway occurs when a secreted Wnt ligand binds to the heterodimeric receptor complex of a frizzled (Fzds) and single transmembrane lipoprotein receptor-related protein (LPR5/6) and deactivates the destruction complex. Then, non-phosphorylated-active β-catenin accumulates in the cytoplasm and later translocates into the nucleus where it binds to the lymphoid enhancer factor/T-cell factor (LEF/TCF) and activates Wnt target genes (Figure 1B) [25-27].
Wnt/β-catenin pathway and ccRCC
The canonical Wnt signaling pathway is also connected with the pathogenesis of a wide variety of cancers as well as ccRCC. In diverse cancer types, alteration in the expression of different Wnt ligands, Wnt receptors (Fzds), and Wnt antagonists stimulate the Wnt/β-catenin pathway (Figure 1C) [28].
Role of Wnt ligands in ccRCC
The Wnt ligands have the leading role in the activation of the Wnt signaling cascade is more important than mutations of various genes and components related to the pathway. Tumor cells and a mixture of cells with the tumor microenvironment contributes with the secretion of Wnt ligands to induce the canonical pathway, but the autocrine supply of ligands strongly stimulate the Wnt pathway in several cancers, including ccRCC [29]. Stimulation of the canonical pathway by Wnt ligands and accumulation of β-catenin in the cytoplasm are restricted to ccRCC [30].
In the Wnt ligand family, Wnt1 and Wnt10 are associated with the canonical pathway, whereas Wnt5a and Wnt7 are linked to the non-canonical pathway [31, 32]. Accordingly, the level of Wnt ligands expression and downstream signaling mechanisms differ in various tumors. In ccRCC, high expression of Wnt1 correlates with larger tumor size, advanced stage, and risk of vascular tumor infiltration, but not with overall survival (OS) and cancer-specific survival (CSS) [33]. Augmentation of the Wnt1 is associated with ccRCC progression and enhanced expression of Wnt10A portrays an independent risk factor for carcinogenesis of ccRCC [34]. The majority of ccRCCs show hypermethylated silencing of the Wnt7A gene, which has a positive association with tumor grade and stage [35]. Reduced expression of Wnt5A mRNA was reported in the development of kidney tumors [36]. In humans, the interaction between the Wnt ligands and Fzd receptors is conserved, and cancer type specific. Some Wnt ligands exhibited as a tumor suppressor and some as an oncogene.
Role of Wnt Antagonist in ccRCC
Generally, the Wnt antagonist genes serve as a tumor suppressor and inhibit the Wnt signaling pathway [37]. There are two functional classes of Wnt-antagonists: The class 1, secreted Fzd‑related protein (Sfrp) family and Wnt‑inhibitory factor‑1 (Wif‑1), antagonizes Wnt ligands, whereas class 2, Dickkopf (Dkk) family, interferes with co-receptors lipoprotein receptor-related proteins (Lrp5/6) and Kremen (Krm). In ccRCC, loss of function of the Wnt antagonists, Wif-1, sFRP, Dkk, IGFBP-4, and SOSTDC1, leads to activation of the Wnt singling pathway [38-41]. Sporadic ccRCC frequently expresses low Wif-1 levels, while the restoration of Wif-1 reduces the tumor growth [42]. Several Wnt antagonists were found to be highly methylated (sFRP-1, sFRP-2, sFRP-4, sFRP-5, Wif-1, and Dkk-3); for example, methylated sFRP-1 has recently emerged as an independent predictor of ccRCC, and sFRP-2 and sFRP-4 noted a trend towards significance as independent predictors had been observed [43-45].
In the serum DNA of ccRCC patients, the methylation status of all Wnt antagonist genes is significantly associated with advanced tumor grade and stage [43]. A small set of primary ccRCC showed methylation-associated silencing of SFRP1, but in contrast, metastatic ccRCC overexpressed SFRP1 [46, 47]. Tissue samples from ccRCC patients showed significantly lower Wnt antagonist mRNA and protein levels (WIF1, sFRP1, Dkk1, and Dkk3) [42, 48, 49]. Both tissues and serum samples of ccRCC displayed decreased expression of Dkk1 and Dkk3, while the Dkk1 levels significantly correlated with clinicopathological parameters [49].
In contrast, the expression of DKK4 that negatively regulates Wnt singling in ccRCC, was significantly higher in ccRCC, but did not affect the biological behavior of ccRCC [50]. Another IGFBP-4, an antagonist of the canonical pathway, was markedly higher in metastatic ccRCC than the non-metastatic ccRCC and kidney cortex [51]. Furthermore, in invivo study on ccRCC cells, IGFBP-4 expression induces cell growth, metastasis, Wnt/beta-catenin signaling [51]. The Wnt antagonists induce the Wnt pathway through diverse mechanisms and, in some conditions, inhibit the action of other antagonists. Both serum and tissues of the ccRCC reported an identical pattern of methylation of the Wnt antagonist genes, which implied that Wnt antagonist genes could be a reliable and feasible biomarker for ccRCC staging and prognosis.
Role of Wnt Receptors in ccRCC
The Wnt receptor complex is composed of two components: a member of the frizzled (Fzd) family and low-density-lipoprotein-receptor related proteins (LRP-5 and LRP-6). The Wnt receptor complex mediates the activation of the canonical pathway; however, it is not clear that the non-canonical pathway requires LRP5/LRP6 in the receptor complex for activation.
In humans, ten Fzds (Fzd1-10), 7-transmembrane receptors, are identified, which are nexus with LRP5/6/ ROR2/RYK [22, 52]. Retrospective studies reported that the elevated levels of some Fzd receptors linked with patient’s survival in multiple cancers [53]. The ccRCCs exhibit frequent alterations of the Fzd receptors, and knockdown of Fzds in ccRCC cells diminish the cell growth, invasion, motility, metastasis, and chemoresistance by inhibiting the Wnt pathway [53-55]. Less than 1% of ccRCC patients carry Fzd1 mutation [56]. Sunitinib-resistant ccRCC cell lines and ccRCC patient samples express high levels of Fzd1 mRNA, and the high expression Fzd1 mRNA levels in ccRCC correlate with tumor stage, recurrence, and favorable OS and DFS [56]. The expression of receptor Fzd5 and Fzd8 are significantly higher in ccRCC compared with the kidney cortex, and 30% of the ccRCCs register an association between Fzd5 and nuclear expression of cyclin D1 [54, 57]. Even though ccRCC demonstrate significantly elevated Fzd7 levels than the surrounding kidney tissues, there was no correlation observed with clinicopathological parameters [58].
Tumors unveil a notably higher expression of mRNA and protein levels of the Fzd8 receptor than peritumor tissues. As a result, increased FZD8 receptor, by stimulating both the canonical and non-canonical Wnt/β-catenin pathways, facilitates the proliferation, epithelial to mesenchymal transition (EMT), and metastasis of ccRCC [59]. The Fzd10 interacts with Hypoxia-inducible protein 2 (HIG2) and induce Wnt signaling target genes [60]. The involvement of Fzd receptors with co-receptors, LRP5/6, RYK, and ROR2, also possess a vital function in Wnt signaling, which have indicated the potential targets for cancer treatment.
Role of Wnt in ccRCC
Multiple factors, other than Wnt associated, can modulate the canonical Wnt signaling pathway and contribute to the ontogenesis of ccRCC. The canonical pathway in ccRCC is regulated at the level of β-catenin and co-transcription factors levels. Either inactivation of APC or activation of β-catenin by mutations can induce the Wnt pathway [30]. Isoforms of TCF-4 (T-cell factor-4), a co-transcriptional factor of β-catenin, regulates the progression of ccRCC through inhibition of apoptosis [61]. Receptor tyrosine kinase-like orphan receptor 2 (Ror2) stabilizes β-catenin in response to Wnt3a exogenous signals leading to an activation of the Wnt/β-catenin cascade [62]. Aberrant expression of β-catenin has increased cell proliferation, migration, and invasion, while repression of β-catenin inhibits PCNA (Proliferating cell nuclear antigen) and Ki67 expression, and delays tumor progression by in the rat [63].
Different long non-coding RNAs (lncRNAs) can also modulate the Wnt pathway at transcriptional to post-translational levels [64]. In a ccRCC cell line, the colon cancer-associated transcript 2 (CCAT2) promoted the ccRCC tumorigenesis [65] whereas LINC01510 negatively governed the canonical pathway and suppressed cell proliferation, invasion, EMT and promoted the apoptosis of ccRCC [66].
Furthermore, other factors can also dictate the canonical pathway at different layers. Wnt/β-catenin pathway inhibitors like ethacrynic acid (EA), ciclopirox olamine (CIC), and piroctone olamine (PO) suppressed the progression of ccRCC [67, 68]. Interestingly, an anthelmintic drug Pyrvinium (CK1α-dependent manner), suppressed the progression of ccRCC by induction of apoptosis [69]. A glycolytic enzyme ALDOA (fructose-bisphosphate aldolase A) promoted proliferation, migration, and invasion of ccRCC cells [68]. In addition, ubiquitin ligases can decrease cell invasion and ccRCC progression by stabilizing β-catenin [70, 71].
The activity of β-catenin not only establishes a bridge between Wnt and cadherin pathways but also collaborates in the regulation of EMT, gene expression, and cell adhesion in cancer. E-cadherin, an EMT marker, can constrain the translocation of β-catenin from the membrane to the cytoplasm [72]. In ccRCC patients, the E-cadherin/β-catenin status was found to be an independent survival factor [72]. Methylation induced gene silencing of SOX7 (SRY-related high mobility group box 7) inhibited the cell growth and cell proliferation of ccRCC cells by modulating the Wnt cascade [73].
Various studies reported that miscellaneous factors also regulate the canonical pathway in ccRCC. High expression of leucine-rich protein (NLR) family NLRC5, a novel member of the nucleotide-binding domain, induced the Wnt/β-catenin axis in ccRCC by positively regulating β-catenin [74]. Diminished expression of the CXXC4 gene induced the Wnt signaling pathway and correlated with a more aggressive ccRCC phenotype [75]. A nucleolar protein MSP58 positively regulated the proliferation and invasion of ccRCC through the Wnt/β-catenin axis [76]. Kindlin‑2, known as an integrin-interacting and FERM-domain containing protein, involved in the ccRCC progression through the Wnt pathway [77]. RNA binding protein QK1, through Wnt and GPCR pathway, inhibits the ccRCC proliferation by attuned cell contact inhibition [78].
Impact of VHL and Hypoxia on Wnt/β-catenin Pathway in ccRCC
Sporadic ccRCC is strongly associated with the VHL-HIF signature pathway, due to loss of function of pVHL and stabilization of the oxygen sensor HIF-α. In ccRCC, the canonical Wnt pathway forms a complex network with the VHL-HIF signaling pathway [79]. In normal conditions, pVHL serves as an E3-ligase adaptor protein and targets HIF-a subunits for degradation by ubiquitination [80, 81]. In ccRCC, double deletion of the VHL gene stabilizes the HIF-α subunits, which in turn promotes cell proliferation, metastasis, EMT, and angiogenesis [82]. Inactivation of VHL, along with other tumor suppressor genes, can cause ccRCC oncogenesis and also regulated the canonical Wnt signaling pathway [79, 83].
In ccRCC, the short arm of chromosome 3 is frequently affected by somatic alterations in regions where both the VHL and the β-catenin coding genes are located [84, 85]. Mutation of the β-catenin coding gene is uncommon in ccRCC, but alternate mechanisms might cause activation of β-catenin through the HIF-dependent or HIF-independent pathways or via growth factors [30, 86, 87]. Aberrant expression of VHL enabled the oncogenic β-catenin pathway through the mediation of hepatocyte growth factor- β (HGF-β) [14]. In addition, VHL deficient ccRCC cells prompted β-catenin–driven transcription of AURKA, which was associated with the deformation of primary cilia and induced renal oncogenesis [88]. In sporadic ccRCC, the expression of VHL and JADE-1 was lower; however, in normal conditions, pVHL stabilized JADE-1 that negatively regulated Wnt/β-catenin and promoted apoptosis and renal tumor suppression [89, 90].
Consolidated activation of HIF-α-β-catenin signal induces the canonical Wnt/β-catenin pathway in the absence of VHL [91]. The role of HIF-1α confined to specific tumor types while regulating the Wnt-β-catenin pathway [92]. In hypoxic ccRCC, HIF-2α directly bound to β-catenin and enhanced the transcriptional activity of β-catenin/TCF by recruiting the transcriptional co-activator p300, and contributed to tumor growth, angiogenesis, metastasis, and dedifferentiation of tumor cells [93].
These updated reports on the Wnt/ β-catenin pathway and its collaboration with the VHL-HIF-α pathway dictate fundamental cellular functions mediated by complex responses. These pathways are essential for genomic instability, hypoxia response, DNA repair, epigenetic modification, splicing, and other cellular processes. Knowledge of aberrant signaling of these pathways contributes to the additional roadmap for the ccRCC carcinogenesis, which can lead to novel therapies in the future.
Summary
In normal physiological conditions, different signaling pathways contribute to the development and cellular homeostasis. In cancer, the critical components of the signaling pathways are often distorted, leading to dysregulation. In ccRCC, the role of the Wnt/ β-catenin pathway in the formation and progression of ccRCC is still elusive. In previous decades, targeting kinase receptors and key signaling components provided improved survival rates. Besides, the simultaneous effect of recent immunotherapies along with tyrosine kinase inhibitors provide a better response. For effective treatments, it is essential to gain in-depth knowledge of the multiple components of the signaling pathways and their interaction and role in the pathogenesis of ccRCC.
Funding
This work was supported by the Cancerforskningsfonden i Norrland/ Lions Cancerforskningsfond - AMP19-976 and AMP20-1009 (RTS).
Article Info
Article Type
Review ArticlePublication history
Received: Mon 16, Mar 2020Accepted: Thu 02, Apr 2020
Published: Fri 10, Apr 2020
Copyright
© 2023 Raviprakash T. Sitaram. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.03.09
Author Info
Börje Ljungberg Göran Roos Marene Landström Raviprakash T. Sitaram
Corresponding Author
Raviprakash T. SitaramDepartment of Medical Biosciences, Pathology, Translation Research Center (TRC), Umeå University, SE-901 85 Umeå, Sweden
Figures & Tables
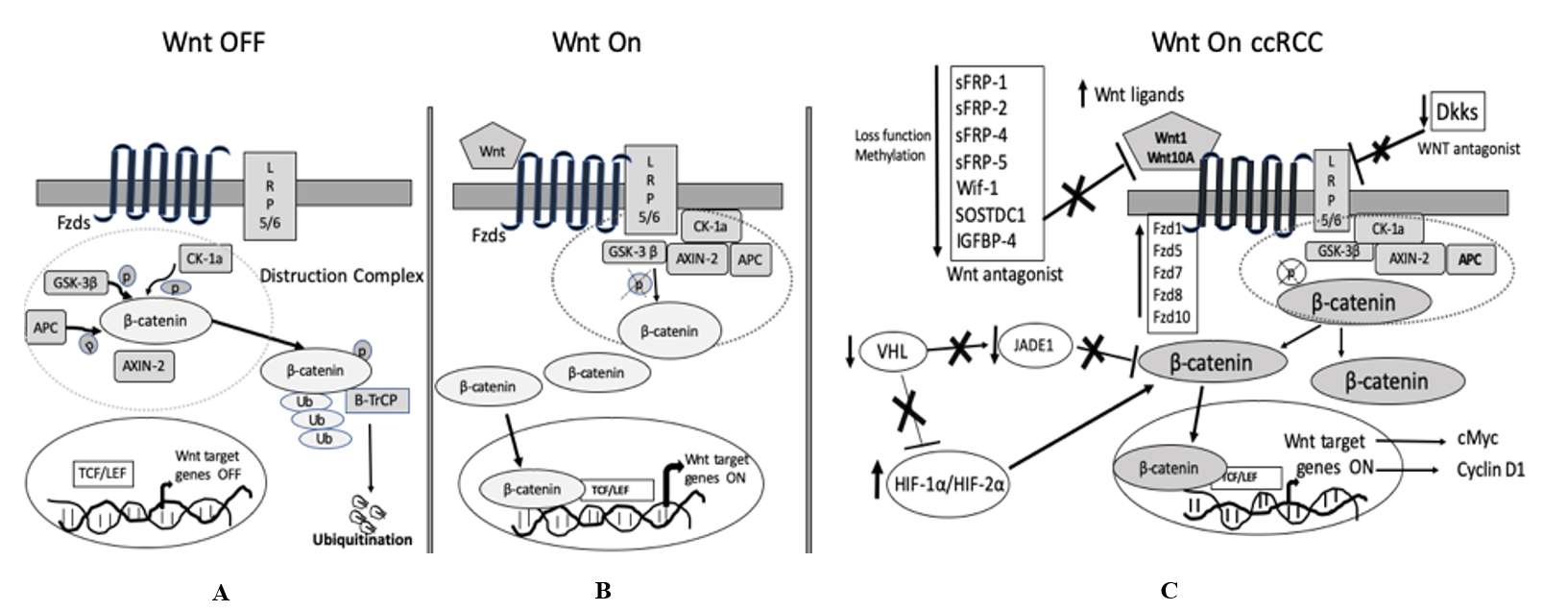
A) Wnt-OFF. When Fzd/LRP receptors are not engaged, the destruction complex [in the dotted circle: CK-1, GSK-3β, APC, and Axin-1] phosphorylates β-catenin at various residues and destabilizes β-catenin. Consequently, β-catenin undergoes proteasomal degradation.
B) Wnt-ON. Binding of Wnt ligands trigger membrane receptors Fzd /LRP and deactivate the destruction complex; as a result, stabilized β-catenin accumulate in the cytoplasm and then translocated into the nucleus to enhance the transcription of the Wnt target genes with co-factor TCF/LEF.
C) Wnt-in ccRCC. In ccRCC, secreted Wnt ligands bind to Wnt membrane receptors, or loss of function/ deletion/ promotor hypermethylation of Wnt antagonists or elevated expression of Fzds disrupt the destruction complex (in the dotted circle). Subsequently, non-phosphorylated β-catenin translocate into the nucleus, and in association with TCF/LEF stimulate Wnt/ β-catenin pathway and further activate Wnt target genes like cMYC, cyclin D1; Loss of pVHL in ccRCC activates Wnt/ β-catenin pathway not only by stabilizing HIF-1α but also by destabilizing JADE1.
References
- Siegel RL, KD Miller, A Jemal (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7-30. [Crossref]
- Eble JN, Sauter G, EPstein JI, Sesterhenn IA (2004) Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press.
- Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M et al. (2018) The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep 23: 313-326. [Crossref]
- Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27: 612-24. [Crossref]
- Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J et al. (2005) Histological subtyping and nuclear grading of renal cell carcinoma and their implications for survival: a retrospective nation-wide study of 629 patients. Eur Urol 48: 593-600. [Crossref]
- Kovacs G (1996) Molecular genetics of human renal cell tumours. Nephrol Dial Transplant 11: 62-65. [Crossref]
- Chen M, Ye Y, Yang H, Tamboli P, Matin S et al. (2009) Genome-wide profiling of chromosomal alterations in renal cell carcinoma using high-density single nucleotide polymorphism arrays. Int J Cancer 125: 2342-2348. [Crossref]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH et al. (1994) Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7: 85-90. [Crossref]
- Cancer Genome Atlas Research Network (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499: 43-49. [Crossref]
- Herman JG, Latif F, Weng Y, Lerman MI, Zbar B et al. (1994) Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A 91: 9700-9704. [Crossref]
- Batavia AA, P Schraml, H Moch (2019) Clear cell renal cell carcinoma with wild-type von Hippel-Lindau gene: a non-existent or new tumour entity? Histopathology 74: 60-67. [Crossref]
- Jonasch E, J Gao, WK Rathmell (2014) Renal cell carcinoma. BMJ 349: g4797. [Crossref]
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ et al. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427-434. [Crossref]
- Peruzzi B, G Athauda, DP Bottaro (2006) The von Hippel-Lindau tumor suppressor gene product represses oncogenic beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci U S A 103: 14531-14536. [Crossref]
- Clevers H, R Nusse (2012) Wnt/beta-catenin signaling and disease. Cell 149: 1192-1205. [Crossref]
- Clevers, H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469-480. [Crossref]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R (1997) beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797-3804. [Crossref]
- Cavard C, Colnot S, Audard V, Benhamouche S, Finzi L et al. (2008) Wnt/beta-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol 4: 647-660. [Crossref]
- Reya T, H Clevers (2005) Wnt signalling in stem cells and cancer. Nature 434: 843-850. [Crossref]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R et al. (1999) The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 9: 207-210. [Crossref]
- Liu C, Li Y, Semenov M, Han C, Baeg GH et al. (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837-847. [Crossref]
- Logan CY, R Nusse (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781-810. [Crossref]
- Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K et al. (1998) Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells 3: 395-403. [Crossref]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D et al. (1996) The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443-1454. [Crossref]
- Ilyas M (2005) Wnt signalling and the mechanistic basis of tumour development. J Pathol 205: 130-144. [Crossref]
- Katoh M, M Katoh (2007) WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13: 4042-4045. [Crossref]
- Wu D, W Pan (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35: 161-168. [Crossref]
- Xu Q, Mirja Krause, Anatoly Samoylenko, Seppo Vainio (2016) Wnt Signaling in Renal Cell Carcinoma. Cancers (Basel) 8: 57 [Crossref]
- Barker N, H Clevers (2006) Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov 5: 997-1014. [Crossref]
- Kim YS, Kang YK, Kim JB, Han SA, Kim KI et al. (2000) beta-catenin expression and mutational analysis in renal cell carcinomas. Pathol Int 50: 725-730. [Crossref]
- Shimizu H, Julius MA, Giarré M, Zheng Z, Brown AM et al. (1997) Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8: 1349-1358. [Crossref]
- Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E et al. (2009) Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 8: 90. [Crossref]
- Kruck S, Eyrich C, Scharpf M, Sievert KD, Fend F et al. (2013) Impact of an altered Wnt1/beta-catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int J Mol Sci 14: 10944-10957. [Crossref]
- Hsu RJ, Ho JY, Cha TL, Yu DS, Wu CL et al. (2012) WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/beta-catenin pathway. PLoS One 7: e47649. [Crossref]
- Kondratov AG, Kvasha SM, Stoliar LA, Romanenko AM, Zgonnyk YM et al. (2012) Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One 7: e47012. [Crossref]
- Tamimi Y, Ekuere U, Laughton N, Grundy P (2008) WNT5A is regulated by PAX2 and may be involved in blastemal predominant Wilms tumorigenesis. Neoplasia 10: 1470-1480. [Crossref]
- Kawano Y, R Kypta (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116: 2627-2634. [Crossref]
- Hirata H, Hinoda Y, Majid S, Chen Y, Zaman MS et al. (2011) DICKKOPF-4 activates the noncanonical c-Jun-NH2 kinase signaling pathway while inhibiting the Wnt-canonical pathway in human renal cell carcinoma. Cancer 117: 1649-1660. [Crossref]
- Hirata H, Hinoda Y, Nakajima K, Kikuno N, Yamamura S et al. (2009) Wnt antagonist gene polymorphisms and renal cancer. Cancer 115: 4488-4503. [Crossref]
- Kawakami K, Yamamura S, Hirata H, Ueno K, Saini S et al. (2011) Secreted frizzled-related protein-5 is epigenetically downregulated and functions as a tumor suppressor in kidney cancer. Int J Cancer 128: 541-550. [Crossref]
- Saini S, S Majid, R Dahiya (2011) The complex roles of Wnt antagonists in RCC. Nat Rev Urol 8: 690-699. [Crossref]
- Kawakami K, Hirata H, Yamamura S, Kikuno N, Saini S et al. (2009) Functional significance of Wnt inhibitory factor-1 gene in kidney cancer. Cancer Res 69: 8603-8610. [Crossref]
- Urakami S, Shiina H, Enokida H, Hirata H, Kawamoto K et al. (2006) Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res 12: 6989-6997. [Crossref]
- Morris MR, Ricketts C, Gentle D, Abdulrahman M, Clarke N et al. (2010) Identification of candidate tumour suppressor genes frequently methylated in renal cell carcinoma. Oncogene 29: 2104-2117. [Crossref]
- Kawamoto K, Hirata H, Kikuno N, Tanaka Y, Nakagawa M et al. (2008) DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. Int J Cancer 123: 535-542. [Crossref]
- Awakura Y, Nakamura E, Ito N, Kamoto T, Ogawa O (2008) Methylation-associated silencing of SFRP1 in renal cell carcinoma. Oncol Rep 20: 1257-1263. [Crossref]
- Saini S, Liu J, Yamamura S, Majid S, Kawakami K et al. (2009) Functional significance of secreted Frizzled-related protein 1 in metastatic renal cell carcinomas. Cancer Res 69: 6815-6822. [Crossref]
- Gumz ML, Zou H, Kreinest PA, Childs AC, Belmonte LS et al. (2007) Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 13: 4740-4749. [Crossref]
- Guo CC, Zhang XL, Yang B, Geng J, Peng B et al. (2014) Decreased expression of Dkk1 and Dkk3 in human clear cell renal cell carcinoma. Mol Med Rep 9: 2367-2373. [Crossref]
- Zhai W, Hu GH, Zheng JH, Peng B, Liu M et al. (2014) High expression of the secreted protein dickkopf homolog 4: roles in invasion and metastasis of renal cell carcinoma and its association with Von Hippel-Lindau gene. Int J Mol Med 33: 1319-1326. [Crossref]
- Ueno K, Hirata H, Majid S, Tabatabai ZL, Hinoda Y et al. (2011) IGFBP-4 activates the Wnt/beta-catenin signaling pathway and induces M-CAM expression in human renal cell carcinoma. Int J Cancer 129: 2360-2369. [Crossref]
- He X, Semenov M, Tamai K, Zeng X et al. (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131: 1663-1677. [Crossref]
- Ueno K, Hirata H, Hinoda Y, Dahiya R et al. (2013) Frizzled homolog proteins, microRNAs and Wnt signaling in cancer. Int J Cancer 132: 1731-1740. [Crossref]
- Furge KA, Chen J, Koeman J, Swiatek P, Dykema K et al. (2007) Detection of DNA copy number changes and oncogenic signaling abnormalities from gene expression data reveals MYC activation in high-grade papillary renal cell carcinoma. Cancer Res 67: 3171-3176. [Crossref]
- Majid S, S Saini, R Dahiya (2012) Wnt signaling pathways in urological cancers: past decades and still growing. Mol Cancer 11: 7. [Crossref]
- Peng Q, Wang L, Zhao D, Lv Y, Wang H et al. (2019) Overexpression of FZD1 is Associated with a Good Prognosis and Resistance of Sunitinib in Clear Cell Renal Cell Carcinoma. J Cancer 10: 1237-1251. [Crossref]
- Janssens N, Andries L, Janicot M, Perera T, Bakker A (2004) Alteration of frizzled expression in renal cell carcinoma. Tumour Biol 25: 161-171. [Crossref]
- Xu R, Zeng S, Xie W, Sun C, Chen YL et al. (2016) The expression and function of Frizzled-7 in human renal cell carcinoma. Clin Transl Oncol 18: 269-276. [Crossref]
- Yang Q, Wang Y, Pan X, Ye J, Gan S et al. (2017) Frizzled 8 promotes the cell proliferation and metastasis of renal cell carcinoma. Oncotarget 8: 78989-79002. [Crossref]
- Thevenod F, PK Chakraborty (2010) The role of Wnt/beta-catenin signaling in renal carcinogenesis: lessons from cadmium toxicity studies. Curr Mol Med 10: 387-404. [Crossref]
- Shiina H, Igawa M, Breault J, Ribeiro-Filho L, Pookot D et al. (2003) The human T-cell factor-4 gene splicing isoforms, Wnt signal pathway, and apoptosis in renal cell carcinoma. Clin Cancer Res 9: 2121-2132. [Crossref]
- Rasmussen NR, Wright TM, Brooks SA, Hacker KE, Debebe Z et al. (2013) Receptor tyrosine kinase-like orphan receptor 2 (Ror2) expression creates a poised state of Wnt signaling in renal cancer. J Biol Chem 288: 26301-26310. [Crossref]
- Yang CM, Ji S, Li Y, Fu LY, Jiang T et al. (2017) beta-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco Targets Ther 10: 711-724. [Crossref]
- Zarkou V, Alexandros Galarasac, Antonis Giakountisad, Pantelis Hatzis (2018) Crosstalk mechanisms between the WNT signaling pathway and long non-coding RNAs. Noncoding RNA Res 3: 42-53. [Crossref]
- Huang JL, Liao Y, Qiu MX, Li J, An Y (2017) Long non-coding RNA CCAT2 promotes cell proliferation and invasion through regulating Wnt/beta-catenin signaling pathway in clear cell renal cell carcinoma. Tumour Biol 39: 1010428317711314. [Crossref]
- Ma B, Zhang J, Zhou W, Chu C, Zhao C et al. (2018) LINC01510 suppresses cell proliferation and invasion by inhibiting Wnt/beta-catenin signaling in renal cell carcinoma. Biochem Biophys Res Commun 505: 7-12. [Crossref]
- VON Schulz-Hausmann SA, Schmeel LC, Schmeel FC, Schmidt-Wolf IG (2014) Targeting the Wnt/beta-catenin pathway in renal cell carcinoma. Anticancer Res 34: 4101-4108. [Crossref]
- Huang Z, Hua Y, Tian Y, Qin C, Qian J et al. (2018) High expression of fructose-bisphosphate aldolase A induces progression of renal cell carcinoma. Oncol Rep 39: 2996-3006. [Crossref]
- Cui L, J Zhao, J Liu (2018) Pyrvinium Sensitizes Clear Cell Renal Cell Carcinoma Response to Chemotherapy Via Casein Kinase 1alpha-Dependent Inhibition of Wnt/beta-Catenin. Am J Med Sci 355: 274-280. [Crossref]
- Zhao W, Zhou J, Deng Z, Gao Y, Cheng Y (2016) SPOP promotes tumor progression via activation of beta-catenin/TCF4 complex in clear cell renal cell carcinoma. Int J Oncol 49: 1001-1008. [Crossref]
- Wen JL, Wen XF, Li RB, Jin YC, Wang XL et al. (2015) UBE3C promotes growth and metastasis of renal cell carcinoma via activating Wnt/beta-catenin pathway. PLoS One 10: e0115622. [Crossref]
- Zhang X, Yang M, Shi H, Hu J, Wang Y et al. (2017) Reduced E-cadherin facilitates renal cell carcinoma progression by WNT/beta-catenin signaling activation. Oncotarget 8: 19566-19576. [Crossref]
- Wang L, Fan Y, Zhang L, Li L, Kuang G et al. (2019) Classic SRY-box protein SOX7 functions as a tumor suppressor regulating WNT signaling and is methylated in renal cell carcinoma. FASEB J 33: 254-263. [Crossref]
- Wang Q, Ding H, He Y, Li X, Cheng Y et al. (2019) NLRC5 mediates cell proliferation, migration, and invasion by regulating the Wnt/beta-catenin signalling pathway in clear cell renal cell carcinoma. Cancer Lett 444: 9-19. [Crossref]
- Kojima T, Shimazui T, Hinotsu S, Joraku A, Oikawa T et al. (2009) Decreased expression of CXXC4 promotes a malignant phenotype in renal cell carcinoma by activating Wnt signaling. Oncogene 28: 297-305. [Crossref]
- Zhai X, Wu Y, Zhang D, Chong T, Zhao J (2017) Knockdown of MSP58 inhibits the proliferation and metastasis in human renal cell carcinoma cells. Biomed Pharmacother 91: 54-59. [Crossref]
- Li M, Pei X, Wang G, Zhan J, Du J et al. (2017) Kindlin2 promotes clear cell renal cell carcinoma progression through the Wnt signaling pathway. Oncol Rep 38: 1551-1560. [Crossref]
- Zhu Z, Wei D, Li X, Wang F, Yan F et al. (2019) RNA-binding protein QKI regulates contact inhibition via Yes-associate protein in ccRCC. Acta Biochim Biophys Sin (Shanghai) 51: 9-19. [Crossref]
- Hou W, Z Ji (2018) Generation of autochthonous mouse models of clear cell renal cell carcinoma: mouse models of renal cell carcinoma. Exp Mol Med 50: 30. [Crossref]
- Frew IJ, W Krek (2008) pVHL: a multipurpose adaptor protein. Sci Signal 1: pe30. [Crossref]
- Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC et al. (1999) Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci U S A 96: 12436-12441. [Crossref]
- Frew IJ, H Moch (2015) A clearer view of the molecular complexity of clear cell renal cell carcinoma. Annu Rev Pathol 10: 263-89. [Crossref]
- Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV et al. (2008) Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol 10: 1208-1216. [Crossref]
- Kraus C, Liehr T, Hülsken J, Behrens J, Birchmeier W et al. (1994) Localization of the human beta-catenin gene (CTNNB1) to 3p21: a region implicated in tumor development. Genomics 23: 272-274. [Crossref]
- Maestro ML, del Barco V, Sanz-Casla MT, Moreno J, Adrover E et al. (2000) Loss of heterozygosity on the short arm of chromosome 3 in renal cancer. Oncology 59: 126-130. [Crossref]
- Bilim V, Kawasaki T, Katagiri A, Wakatsuki S, Takahashi K et al. (2000) Altered expression of beta-catenin in renal cell cancer and transitional cell cancer with the absence of beta-catenin gene mutations. Clin Cancer Res 6: 460-466. [Crossref]
- Zeitlin BD, LM Ellis, JE Nor (2009) Inhibition of Vascular Endothelial Growth Factor Receptor-1/Wnt/{beta}-catenin Crosstalk Leads to Tumor Cell Death. Clin Cancer Res 15: 7453-7455. [Crossref]
- Dere R, Perkins AL, Bawa-Khalfe T, Jonasch D, Walker CL et al. (2015) beta-catenin links von Hippel-Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma. J Am Soc Nephrol 26: 553-564. [Crossref]
- Xiao-Fen W, Ting C, Jie L, Deng-Yang M, Qing-Feng Z et al. (2016) Correlation analysis of VHL and Jade-1 gene expression in human renal cell carcinoma. Open Med (Wars) 11: 226-230. [Crossref]
- Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C et al. (2002) The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem 277: 39887-39898. [Crossref]
- Linehan WM, JS Rubin, DP Bottaro (2009) VHL loss of function and its impact on oncogenic signaling networks in clear cell renal cell carcinoma. Int J Biochem Cell Biol 41: 753-756. [Crossref]
- Kaidi A, AC Williams, C Paraskeva (2007) Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol 9: 210-217. [Crossref]
- Choi H, Chun YS, Kim TY, Park JW et al. (2010) HIF-2alpha enhances beta-catenin/TCF-driven transcription by interacting with beta-catenin. Cancer Res 70: 10101-10111. [Crossref]
