Journals
Role of Circulating DNA in the Precocious Diagnosis of Lymphoma: an example in a Transplant-Related Aggressive Lymphoma
A B S T R A C T
We report the case of an aggressive non-Hodgkin lymphoma transmission from a live renal donor to her recipient. Soon after donation, the donor developed a high-grade B cell lymphoma, and the disease became evident also in the recipient three months thereafter, showing the same biological characteristics as confirmed by IgH rearrangement, karyotype and chimerism analyses. In the recipient, the IgH clone appeared in circulating cell free DNA (cfDNA), but not in peripheral blood nor in bone marrow, one month earlier than the imaging evidence of disease.
This case supports the fundamental predictive role of molecular analysis of the plasma compartment in the diagnosis and putatively follow up of lymphomas
Keywords
Circulating DNA, high grade B-cell lymphoma, transplant-related lymphoma, donor cancer transmission, precocious biomarker
Introduction
Solid-organ transplantation inherently carries the risk of disease transmission, as infections or malignancies, although donor cancer transmission (DCT) is a rare phenomenon (0.01%-0.05%) [1]. In kidney transplantation, transmission of lymphoma accounts for approximately 14% of all cases of DCTs, requiring drastic treatment modalities, i.e. graft nephrectomy, withdrawal of the immunosuppressive therapy, and systemic chemotherapy, necessary in about half of cases [2, 3]. Non-Hodgkin’s lymphomas (NHLs) represent 3% of all neoplasms in adults, and diffuse large B-cell lymphomas (DLBCL) account for approximately one third of NHLs. Each DLBCL is characterized by a specific rearrangement of the immunoglobulin heavy chain gene (IgH), making its clonality assessment a reliable marker of minimal residual disease (MRD) during treatment and follow-up. Usually, IgH rearrangements are assessed on bone marrow (BM) samples; indeed, BM represents the standard compartment for follow up because of its better demonstrated sensitivity (about one logarithm higher) [4]. However, ongoing trials (such as the FIL FOLL12 trial - trial.gov identifier number: NCT02063685) are aimed at demonstrating the less-invasive peripheral blood (PB) samples to be comparably sensitive. Additionally, MRD may be assessed from plasma fraction, thus representing a further promising option. Intriguingly, higher plasmatic tumor cell-free DNA (cfDNA) levels at diagnosis have also been correlated with more advanced stage of disease, higher lactate dehydrogenase levels, and shorter progression-free survival [5]. This confers a prognostic, other than diagnostic, potential to this marker, as recently reported in an authoritative review [6]. Moreover, specific clonal IgH rearrangements are detected in 80% of patients at diagnosis by cfDNA analysis and predict clinical relapse earlier than 18F- fluorodeoxyglucose PET/CT (Positron Emission Tomography/Computed Tomography) scan positivization in most of cases [7]. Furthermore, circulating tumor DNA levels significantly predicted clinical response to treatment and disease progression [8]. More recently, digital droplet PCR was shown to be sensitive enough in detecting some lymphoma-associated mutations (e.g. XPO1, EZH2, and MYD88) on cfDNA, thus representing a good tool to monitor new emerging clones or subclones [9].
Case Report
In May 2015, a 30-year-old male with end-stage renal disease secondary to vesico-ureteral reflux underwent live donor renal transplantation from his mother. Extensive pre-transplant screening for donor malignancies, including CT scan, resulted negative. Then, right kidney was collected through hand-assisted laparoscopy. In retrospect, no abnormality was noticed during the surgery at the pancreas head/duodenum level, despite it later represented the first evidenced localization of lymphoma. Although the prompt recovery, the donor complained of mild dyspepsia at a scheduled follow-up visit, two weeks after donation. Laboratory tests showed increased lactate dehydrogenase, while amylase and lipase levels were 274 IU/L and 1100 IU/L, respectively. Subsequent contrast-enhanced CT imaging of the abdomen revealed an uncharacterized, poorly vascularized 6-cm mass adherent to head and uncinated process of the pancreas that dislocated and compressed adjacent structures. She was then admitted to our hospital to perform further diagnostic procedures. Magnetic resonance imaging confirmed previous findings and revealed thickening of the gastric wall associated with multiple retroperitoneal and mesenteric lymphadenopathies. PET scan found them increased in fluorodeoxyglucose uptake; mild peri-splenic and peri-hepatic effusions were present, too.
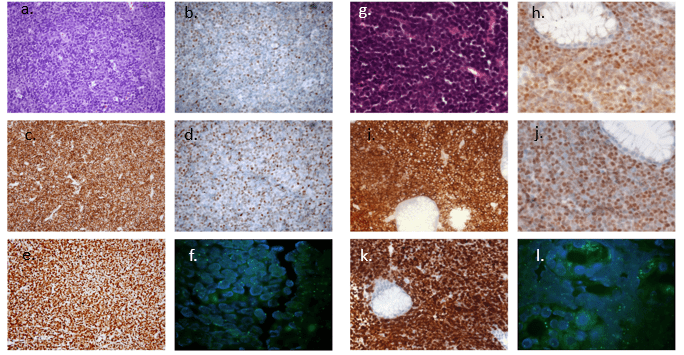
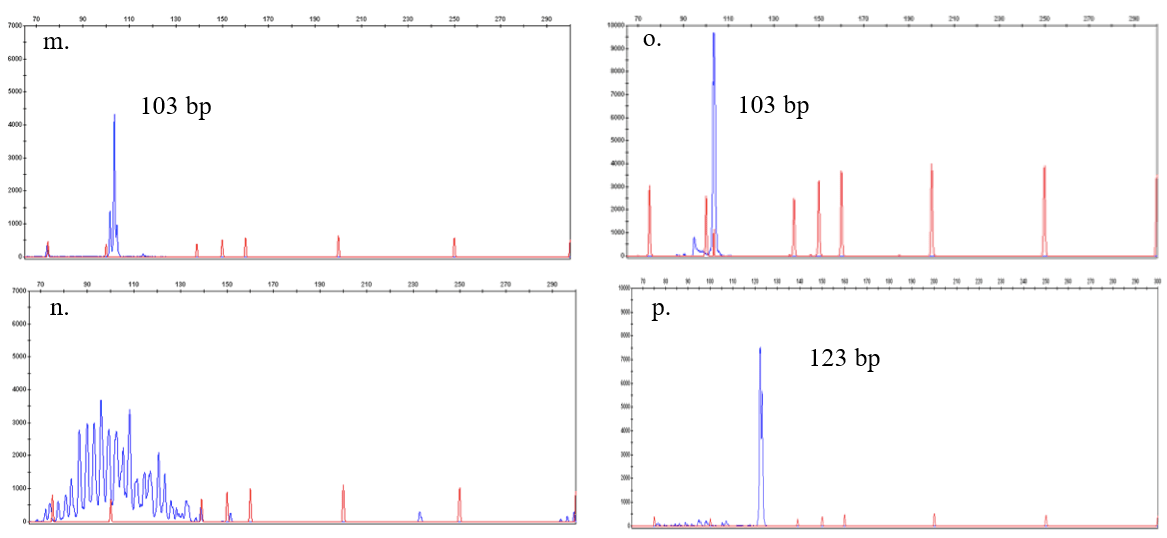
Gastric biopsy results were indicative of unclassifiable B-cell lymphoma, with intermediate features between DLBCL and Burkitt’s lymphoma (Figure 1, g), in regard to the 2008 World Health Organization (WHO) classification of lymphoid neoplasms. After its 2016 revision, the most proper definition for the disease in point has become high grade B-cell lymphoma. Immunohistochemistry resulted negative for CD3, CD5, cyclin-D1, EBV-LMP1, MUM1/IRF4 and TdT, but positive for BCL6, BCL2, c-MYC (Figure 1, h, i, j respectively), CD20 and CD10. Ki67 proliferative index was higher than 90% (Figure 1, k), thus a diagnosis of “triple-expressing” aggressive B-cell lymphoma was made. A CT scan performed for staging purposes revealed disease spreading to both sides of the diaphragm and evident liver involvement. Diagnosis was further confirmed by punch biopsy of a subcutaneous nodule appeared on her right flank nine days after admission. Fluorescent qualitative PCR for IgH clonality performed on both gastric biopsy and subcutaneous nodule revealed two different amplification products: a 103 bp and a 123 bp amplification products, respectively (Figure 1, o and p), suggesting the existence of two different clones. However, IgH clonality analysis of BM and cfDNA identified the 103 bp clone only. Hence, the patient was treated with the R-CODOXM/R-IVAC chemotherapy regimen, achieving complete response [10]. After the donor diagnosis, the recipient underwent PET/CT scan, which was free from possible neoplastic involvement, and evaluation of IgH rearrangements resulted polyclonal on both PB and cfDNA. However, a 103 bp clone, identical to that previously found in the mother, was evidenced by cfDNA analysis two months later (Figure 1, m). Conversely, PB persisted polyclonal (Figure 1, n) and PET/TC scan negative.
After one month, a further PET/CT scan revealed confluent hypermetabolic masses in peri-graft position, extensive abdominal lymphadenopathies, and several bone lesions (Figure 2), all indicative of disease localizations. A lymphoma subtype identical to the mother’s one was then diagnosed through peri-renal core-needle biopsy (Figure 1, a-f and g-l respectively). Histopathology, flow-cytometry, and molecular analyses of BM evidenced no disease involvement. IgH clonality test on lymphoma revealed two different clones, a 103-bp long clone predominating over a 123-bp long one. Both were identical to those previously detected in the two different tumor sites of the donor. Fluorescent in situ-hybridization (FISH) for X and Y chromosomes proved the tumor to be entirely XX (Figure 1, f and l); additionally, short tandem repeats (STR) analysis identifies no recipient-specific sequences, thus strongly suggesting a donor origin. Immunosuppression therapy was immediately withdrawn, and the patient underwent systemic treatment according to the R-CODOXM/R-IVAC scheme, achieving complete response [10]. At the end of the treatment, in January 2016, the patient achieved complete remission, reaching good performance status and stable transplanted-kidney function, maintained with daily prednisone as immunosuppressive therapy to date [11].
At the end of treatment, IgH rearrangements were polyclonal on both PB and plasma. At the last follow-up visit, performed 18 months after the end of therapy, both PET/CT scan and BM evaluation excluded any further disease localization, thus confirming the ongoing complete remission status.
Discussion
We describe the transmission of a highly aggressive B-cell non-Hodgkin lymphoma by kidney transplantation. Remarkably, there was no evidence at the time of organ collection of any donor disease and the lag time between kidney transplantation and lymphoma diagnosis in the recipient was very short. A possible explanation is that tumor cells circulating in the kidney at the time of renal vascular ligation were transmitted to the recipient along with the graft. Due to immunosuppressive therapy, transplanted lymphoma cells escaped immune surveillance, increased in sufficient numbers to present as a perirenal mass and spread to the peripheral blood and other sites. This might explain why cfDNA testing in the recipient was negative at the time of donor’s diagnosis and became positive two months later. In this case, cfDNA detection was used with concurrent PET/CT scans at specified times to monitor disease progression and response to therapy. A recent study showed that repeated cfDNA analysis in follow up identifies patients at risk of recurrence before clinical evidence of disease [12]. Thus, interim cfDNA test is a promising biomarker to identify patients at risk of treatment failure and disease progression [6]. Although that study was conducted on untreated DLBCL patients, cfDNA determination may have a role as a biomarker in high grade B-cell lymphomas, too. This could be particularly true for double- and triple-hit lymphomas, two recently identified subtypes of DLBCL characterized by MYC overexpression and concurrent BCL2 and/or BCL6 translocations. They are associated with poor prognosis and poor efficacy of R-CHOP regimen with shorter overall survival [13]. The MYC-driven nature and the propensity for central nervous system involvement of triple-hit lymphomas has prompted the use of intensified chemotherapy regimens known to be successful in Burkitt’s lymphoma. A large multi-center study found that patients treated with intensive induction regimen had a better overall survival; in particular, treatment with dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA-EPOCH-R) or with cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine with rituximab (R-CODOXM/R-IVAC) was found to give better disease control [14, 15]. Since no consensus guidelines exist, given the high proliferative index (Ki67>90%) and the patient immune impairment, he was treated with a full course of 2 cycles of R-CODOXM/R-IVAC with intrathecal prophylaxis and associated withdrawal of immunosuppression.
Figure 1: Donor and recipient lymphomas characterization and comparation. Figures a-l show immunohistochemistry and FISH analysis for X and Y chromosomes on lymphoma biopsies in donor (a-f) and recipient (g-l); figures m-o represent fluorescent PCR analysis for IgH rearrangement on different samples (DNA ladder for reference length in red, amplified sample DNA in blu).
a. hematoxylin & eosin staining of the donor’s gastric biopsy
b. Bcl6 staining of the donor’s gastric biopsy
c. Bcl2 staining of the donor’s gastric biopsy
d. c-Myc staining of the donor’s gastric biopsy
e. Ki-67 staining of the donor’s gastric biopsy
f. FISH for X and Y chromosomes of the donor’s gastric biopsy: the sample was female (FISH positive for XX)
g. hematoxylin & eosin staining of the recipient lymphoma
h. Bcl6 staining of the recipient lymphoma
i. Bcl2 staining of the recipient lymphoma
j. c-Myc staining of the recipient lymphoma
k. Ki-67 staining of the recipient lymphoma
l. FISH for X and Y chromosomes of the recipient lymphoma: the sample was entirely female (FISH positive for XX)
m. Recipient – at 2 months after the diagnosis of lymphoma in his donor: a clone at 103 bp was detected in cfDNA (DNA isolated from plasma)
n. Recipient – at 2 months after the diagnosis of lymphoma in his donor: the IgH rearrangement performed on lymphocytes from the peripheral blood resulted still polyclonal.;
o. Donor - at the diagnosis of lymphoma: a clone at 103 bp was detected in the plasma (cfDNA), as in the bone marrow and peripheral blood (not shown);
p. Donor - at the diagnosis of lymphoma: a clone at 123 bp was detected in the biopsy of the right flank subcutaneous mass
Figure 2: PET and PET/CT images of the recipient patient, three months after the donor diagnosis.
a. PET/CT scan showing a hypermetabolic peri-renal mass
b. PET scan showing the same peri-renal hypermetabolic activity
c. PET/CT scan where hypermetabolic pelvic lymph nodes are appreciable
d. PET/CT scan showing bone involvement (femur) by the disease
e. Maximum intensity projection (MIP) of PET scan shows both peri-renal and pelvic hypermetabolic activity
Although most cases of DCT are managed with withdrawal of immunosuppression, removal of organ if possible and specific antineoplastic therapy, we avoid transplant nephrectomy preferring a strict serum creatinine monitoring of renal function. The patient eventually preserved graft function and achieved long-term complete remission, thereby confirming what previously shown by Sun et al16. Indeed, they reported an 80% overall response rate, a 60% 2-year progression-free survival, and an 82% overall-survival in patients with “double-hit” lymphomas treated with R-CODOXM/R-IVAC [16, 17].
In summary, the above-reported case raises several noteworthy issues: 1) plasma cfDNA may be a more informative/predictive and less invasive test than BM aspiration to evidence disease onset or recurrence; 2) lymphoma may be transmitted through kidney transplantation, a rare complication that points out challenges in diagnosis and management; 3) aggressive MYC-driven B-cell lymphomas and their diverse mechanisms of immune escape are worth of further clinical and biological investigations.
Acknowledgement
The authors acknowledge Dr. N. De Lio and Prof. F. Vistoli for surgical collaboration with U.B., and Prof. P.A. Erba for imaging analysis.
Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the socially active non-profit organization A.I.L. (Associazione Italiana contro le leucemie-linfomi e mieloma)-Pisa section.
Author Contributions
L.B. and F.R. collected data and wrote the paper; E.O. provided clinical assistance to patients and wrote the paper; M.I.F. performed cytogenetic test and analysis; C.Z. reviewed the literature and wrote the paper; U.B. performed the transplant; E.M.C. performed histological analyses; F.C., E.B. and G.B. assisted the patients and collected data; E.C., F.G., S.Gr. and S.Ga. performed and analyzed molecular tests; M.P. coordinated and supervised the work and wrote the paper.
Article Info
Article Type
Case ReportPublication history
Received: Sat 09, Mar 2019Accepted: Mon 25, Mar 2019
Published: Fri 12, Apr 2019
Copyright
© 2023 Federico Rossari. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.02.02
Author Info
Cristina Zucchinetti Edoardo Benedetti Elena Ciabatti Enrico Orciuolo Eugenio Mario Ciancia Federico Rossari Francesca Guerrini Francesco Caracciolo Gabriele Buda Luca Biavati Maria Immacolata Ferreri Mario Petrini Sara Galimberti Susanna Grassi Ugo Boggi
Corresponding Author
Federico RossariInstitute of Life Sciences, Scuola Superiore Sant’Anna, Pisa, Italy
Figures & Tables
Donor Recipient
a. hematoxylin & eosin staining of the donor’s gastric biopsy
b. Bcl6 staining of the donor’s gastric biopsy
c. Bcl2 staining of the donor’s gastric biopsy
d. c-Myc staining of the donor’s gastric biopsy
e. Ki-67 staining of the donor’s gastric biopsy
f. FISH for X and Y chromosomes of the donor’s gastric biopsy: the sample was female (FISH positive for XX)
g. hematoxylin & eosin staining of the recipient lymphoma
h. Bcl6 staining of the recipient lymphoma
i. Bcl2 staining of the recipient lymphoma
j. c-Myc staining of the recipient lymphoma
k. Ki-67 staining of the recipient lymphoma
l. FISH for X and Y chromosomes of the recipient lymphoma: the sample was entirely female (FISH positive for XX)
m. Recipient – at 2 months after the diagnosis of lymphoma in his donor: a clone at 103 bp was detected in cfDNA (DNA isolated from plasma)
n. Recipient – at 2 months after the diagnosis of lymphoma in his donor: the IgH rearrangement performed on lymphocytes from the peripheral blood resulted still polyclonal.;
o. Donor - at the diagnosis of lymphoma: a clone at 103 bp was detected in the plasma (cfDNA), as in the bone marrow and peripheral blood (not shown);
p. Donor - at the diagnosis of lymphoma: a clone at 123 bp was detected in the biopsy of the right flank subcutaneous mass

a. PET/CT scan showing a hypermetabolic peri-renal mass
b. PET scan showing the same peri-renal hypermetabolic activity
c. PET/CT scan where hypermetabolic pelvic lymph nodes are appreciable
d. PET/CT scan showing bone involvement (femur) by the disease
e. Maximum intensity projection (MIP) of PET scan shows both peri-renal and pelvic hypermetabolic activity
References
- Desai R, Collett D, Watson CJE, Johnson P, Evans T et al. (2014) Estimated risk of cancer transmission from organ donor to graft recipient in a national transplantation registry. Br J Surg 101: 768-774. [Crossref]
- Xiao D, Craig JC, Chapman JR, Dominguez-Gil B, Tong A et al. (2013) Donor cancer transmission in kidney transplantation: A systematic review. Am J Transplant 13: 2645-2652. [Crossref]
- Dziewanowski K, Drozd R, Parczewski M, Klinke M (2014) Multiorgan transplantation from a deceased donor with intravascular diffuse large B-cell lymphoma: transmission of the disease and results of treatment. Clin Transplant 28: 1080-1083. [Crossref]
- Li M, Jia Y, Xu J, Cheng X, Xu C (2017) Assessment of the circulating cell-free DNA marker association with diagnosis and prognostic prediction in patients with lymphoma: a single-center experience. Ann Hematol 96: 1343-1351. [Crossref]
- Papajík T, Jedlicková K, Kriegová E, et al. (2001) Polymerase chain reaction detection of cells carrying t(14;18) in bone marrow of patients with follicular and diffuse large B-cell lymphoma: the importance of analysis at diagnosis and significance of long-term follow-up. Neoplasma 48: 501-505. [Crossref]
- Heitzer E, Haque IS, Roberts CES, Speicher MR (2019) Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 20: 78-88. [Crossref]
- Kurtz DM, Green MR, Bratman S V, Scherer F, Liu CL et al. (2015) Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 125: 3679-3687.[Crossref]
- Roschewski M, Dunleavy K, Pittaluga S, Moorhead M, Pepin F et al. (2015) Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: A correlative biomarker study. Lancet Oncol 16: 541-549. [Crossref]
- Camus V, Sarafan-Vasseur N, Bohers E, Dubois S, Mareschal S et al. (2016) Digital PCR for quantification of recurrent and potentially actionable somatic mutations in circulating free DNA from patients with diffuse large B-cell lymphoma. Leuk Lymphoma 57: 2171-2179. [Crossref]
- Habermann TM, Macon WR, Maurer MJ et al. (2016) Treatment and Clinical Outcomes of High Grade B-Cell Lymphomas with MYC and BCL2 and/or BCL6 Rearrangements (Double Hit/Triple Hit Lymphomas). Blood 128.
- Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R et al. (2016) Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128: 2489-2496. [Crossref]
- Roschewski M, Staudt LM, Wilson WH (2016) Dynamic monitoring of circulating tumor DNA in non-Hodgkin lymphoma. Blood 127: 3127-3132. [Crossref]
- Dunleavy K (2014) Double-hit lymphomas: current paradigms and novel treatment approaches. Hematology Am Soc Hematol Educ Program 2014: 107-112. [Crossref]
- Petrich AM, Gandhi M, Jovanovic B, Czstillo JJ, Rajguru S et al. (2014) Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 124: 2354-2361. [Crossref]
- Cohen JB, Geyer SM, Lozanski G, Zhao W, Heerema NA et al. (2014) Complete response to induction therapy in patients with Myc-positive and double-hit non-Hodgkin lymphoma is associated with prolonged progression-free survival. Cancer 120: 1677-1685. [Crossref]
- Sun H, Savage KJ, Karsan A, Slac GW, Gascoyne RD et al. (2015) Outcome of patients with non-hodgkin lymphomas with concurrent MYC and BCL2 rearrangements treated with CODOX-M/IVAC with rituximab followed by hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk 15:341-348. [Crossref]
- Skopelja-Gardner S, Jones JD, Hamilton BJ, Danilov A V, Rigby WFC (2017) Role for ZAP-70 Signaling in the Differential Effector Functions of Rituximab and Obinutuzumab (GA101) in Chronic Lymphocytic Leukemia B Cells. J Immunol 199: 1275-1282. [Crossref]

