Review of Intersphincteric Resections for Rectal Cancer Treatment
A B S T R A C T
Colorectal cancer is the third most common cancer, second most common cancer in women, and the fourth leading cause of death in the world. Radical surgical treatment with Total Mesorectal Excision (TME) is considered the best treatment for cancer found in the lower third of the rectum and has benefits of complete tumor removal to reduce risk of recurrence and to improve survival. Advances in preoperative chemoradiation therapy have increased chances of achieving a 1 cm distal margin and allowed successful sphincter-preserving surgery by intersphincteric resection (ISR) and Coloanal Anastomosis (CAA) that allows normal defecation. MRI is particularly useful in evaluating localization of the tumor, involvement of anal sphincter (internal and external sphincters), levator ani muscles, and adjacent structures to the anus, with an accuracy of 85%, sensitivity of 87%, and specificity of 75%. Performing ISR with TME oncologic principles achieves similar results to Low Anterior Resection (LAR), but depends on the presence of sufficient Distal Rectal Margin (DRM); if a sufficient DRM cannot be achieved, then patients are offered an Abdominoperineal Resection (APR) with permanent colostomy and poor quality-of-life results.
Keywords
Intersphincteric resection, very low rectal cancer, very low colorectal cancer, distal rection margin, total mesorectal excision
Introduction
Colorectal cancer is the third most common cancer, second most common cancer in women, and fourth leading cause of cancer death in the world [1, 2]. Radical surgical treatment with Total Mesorectal Excision (TME) is considered the best treatment for cancer found in the lower third of the rectum and has benefits of complete tumor removal, to reduce risk of recurrence, and of improved survival [3, 4]. Traditionally, radical treatment of rectal cancer was achieved by total Abdominoperineal Resection (APR) with permanent colostomy, resulting in poor quality of life (QoL) and psychological problems caused by a stoma [1, 3, 5]. The discovery of safe oncological resection margins, termed Distal Resection Margin (DRM), defined by observed maximum distal mural spread of cancer that rarely exceeds 1 cm, and the discovery that such a resection margin can offer sphincter preservation with acceptable continence, has changed the practice of modern surgery [1, 5].
Advances in preoperative chemoradiation therapy have increased chances of achieving a 1 cm distal margin and allowed successful sphincter-preserving surgery by intersphincteric resection (ISR) and Coloanal Anastomosis (CAA) that allows normal defaecation [6-8]. In current surgical practice, sphincter preservation is achieved by Low Anterior Resection (LAR) and ISR; however, ISR achieves an ultra-low DRM of 1 cm by transanal division of the rectum, with partial or total internal anal sphincter resection to achieve adequate oncologic margins and preservation of external anal sphincter [6, 9]. Though ISR eliminates the need for a permanent colostomy, it may require a protective temporal ileostomy [4, 10]. ISR is possible in selected patients with normal anal sphincter tone and lack of tumor invasion in external anal sphincter or levator ani muscles [10]. Performing ISR with TME oncologic principles achieves similar results to LAR but depends on the presence of sufficient DRM; if a sufficient DRM cannot be achieved, then patients are offered an APR with permanent colostomy and poor QoL results [7, 10]. This paper aims to give an overview of ISR and share the principles of the practice at Tata Memorial Hospital, Division of Colorectal Surgery, Mumbai, India [11].
History and Evolution of DRM in ISR
The current treatment of distal rectal cancer in the lower rectum, when located 6 cm above the anal verge, has undergone an evolution. From the extensive resection of APR procedure by Miles et al., it has improved with the introduction of TME by Heald et al. [12, 13]. TME has reduced local recurrence, allowed a DRM of 5 cm, and maintained a circumferential resection margin (CRM) of >1mm, but it still results in a permanent stoma, poor QoL, and psychological trauma [10]. APR has no capacity for sparing anal sphincter to avoid a permanent stoma. The sphincter-preserving procedure of LAR, by Parks et al., allows sphincter preservation with a DRM of 2-5 cm, provided levator ani muscles and sphincter complex are not involved [7, 14].
A breakthrough by Schiessel et al. challenged the lower resection margin of 2 cm, proposing internal anal sphincter resection, DRM of 1-2 cm, and preservation of external sphincter with CAA, with or without a covering temporal ileostomy [15]. This procedure maintains good oncological outcomes, with recurrency rates of 6%, as an alternative to APR and LAR [15]. This breakthrough was possible once researchers realized that local lymphatic spread of a tumor in the distal rectum and direct invasion (extra nodal metastasis) occurred to a greater extent proximally and to a lesser extent distally, sparing a distal length that allows anastomosis with the anal canal [15]. Most authors achieved results of CAA with a DRM of 5 mm, as the DRM does not contraindicate an ISR if R0 resection can be achieved [4, 7-9, 15]. This realization allowed resection of the internal anal sphincter, with acceptable levels of continence and unwanted symptoms, termed LAR syndrome, that result from loss of the rectal reservoir and internal anal sphincter [9, 15]. The internal anal sphincter contributes 55% of anal sphincter continence, while 30% comes from external sphincter and 15% from hemorrhoidal plexus [9].
Most distal rectal cancers are already locally advanced at the time of diagnosis. Treatment by sphincter-preserving surgery with the advent of Neoadjuvant Chemoradiotherapy (NACRCT) gives benefit of reduced distal mural spread (at rate of 1.6%) and reduced recurrence, downstaging the tumor with a pathological complete response rate (pCR) of 10-30%. It also allows sphincter preservation and R0 resection of very low rectal cancer [9, 16]. Before NACRCT, the lymph node and extra nodal metastasis was 7-21%, achievable with a longer interval between NACRCT and surgery, as reported by Guedj et al. and supported by NCCN guidelines of 2020 [16, 17].
Importance of Preoperative Imaging – Endoscopic Ultrasound and MRI
MRI and Endoscopic Ultrasound (EUS) are imaging techniques that are commonly used in staging distal rectal cancer, when evaluating whether the cancer is amenable to ISR, and in addition to evaluation by digital rectal examination and rigid sigmoidoscopy [1, 2, 5]. MRI is used distinguish the lower rectum as the part below the origin of levator ani muscles [2, 5]. Arya et al. have documented that MRI is particularly useful in evaluating localization of the tumor, the involvement of anal sphincter (internal and external sphincters), levator ani muscles, and adjacent structures to the anus, with an accuracy of 85%, sensitivity of 87%, and specificity of 75% [2]. This information is important when deciding whether the distal rectal cancer is treatable with ISR, to allow resection of the internal sphincter and to spare the external sphincter with a curative intent, acceptable anal continency, and QoL [2]. MRI can determine with acceptable accuracy which part of the anal sphincter complex is involved (internal or external sphincter), information required when deciding whether sphincter-preserving surgery is an option [2].
EUS on the other hand is highly accurate, with a sensitivity of 94% and specificity of 86%. It is more accurate than MRI when determining the T1 and T2 stages of distal rectal cancer, while MRI is more accurate to determine T3 and T4 lesions [1, 2]. While Endoscopic Rectal Ultrasound (ERUS) has higher accuracy than MRI for T1 and T2 lesions, Arya et al. report that ERUS is not commonly used in their clinical practice, at less than 1% of cases, compared to MRI: ERUS is not useful in stenosing lesions, the majority of clinical presentations for distal rectal cancer; it cannot reliably evaluate the circumferential resection margin and mesorectal nodes, and it is operator dependent, with high inter-rater reliability at their institution [2]. They state that T-staging of MRI is interpreted in T2W images of MRI that cannot be reliably distinguished between T1 and T2 images [2]. Therefore, high-resolution MRI is their choice for the primary staging of distal rectal cancer [2].
Indications
In most individuals, the distance from anal verge to the lower third of the rectum is between 2-6 cm, and therefore the rule of fours applies: The anal canal should be 4 cm long, while the lower third of the rectum should be 4-8 cm from the anal verge [15]. The indications for ISR only apply to tumors in the lower third of the rectum and therefore include the following:
i. Tumor depth of T1-3, without involvement of external anal sphincter or levator ani muscles.
ii. Tumor depth of T4 invading external anal sphincter or levator ani muscles that have not responded to NACRCT.
iii. Very low tumors less than 2 cm from anal verge.
iv. Anal continence without sphincter functional insufficiency, based on history and physical examination. Manometry is not routinely used in our clinic setting; this practice has been evaluated by other authors [15].
Surgical Technique of ISR
ISR surgical management depends on the American Joint Committee on Cancer (AJCC) clinical stage for distal rectal cancer, confirmed by histopathology and evaluated by imaging, and then the decision of Multidisciplinary Team (MDT) meeting. This team comprises a surgical, medical and radiation oncologist, a radiologist, and a gastroenterologist. The MDT decides whether to offer the patient upfront surgery or NACRCT followed by surgery with curative intent. The surgical technique for ISR, performed in the Division of Colorectal Surgical Oncology at Tata Memorial Center, comprises abdominal and perineal parts. The abdominal part is done as either an open or a laparoscopic procedure. Staging laparotomy or laparoscopy is done before the abdominal stage of the ISR procedure, to rule out diffuse peritoneal disease and liver metastasis. Peritoneal disease and liver metastasis are a sign of advanced metastatic disease. Our recent publication details our experience with a laparoscopic approach, but the operative steps summarized below apply to both open and laparoscopic procedures for ISR [18].
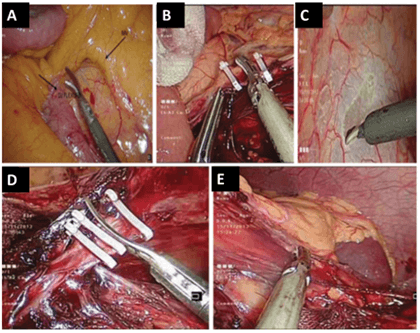
The first phase of the abdominal part, done in the Lloyd Davis position with a head-down tilt of about 10⁰ and left leg up, has an endpoint of inferior mesenteric artery ligation at the origin, mobilization of the left colon, and takedown of splenic flexure, as shown in (Figure 1) below. In the second phase, the TME method described by Heald et al., of sharp dissection between pelvic wall and mesorectal fascia, is performed to mobilize the rectum; the endpoints of levator ani muscle exposure, with dissection entry into the intersphincteric space, are as low as possible down to the pelvic floor, as shown in (Figure 2) below [13]. For the perineal part, the left-up position is neutralized, transanal submucosal purse string (to prevent spillage of fecal matter and tumor cell dissemination) is placed, the Lone Star retractor is attached, and 1 mg of adrenaline diluted in 20 mL of saline is injected submucosally (to minimize bleeding and facilitate intersphincteric dissection). The three types of ISR can be done based on tumor location, relative to dentate line, as partial and subtotal (tumor above dentate line) or total (tumor below dentate line), depending on surgeons’ preference and tumor location.
Figure 1: A) Identification of the inferior mesenteric vein. B) High ligation of the inferior mesenteric vein. C) Scoring of the white line of Toldt. D) High ligation of the inferior mesenteric artery. E) Mobilization of the splenic flexure. Images obtained with permission from Pai et al. [18].
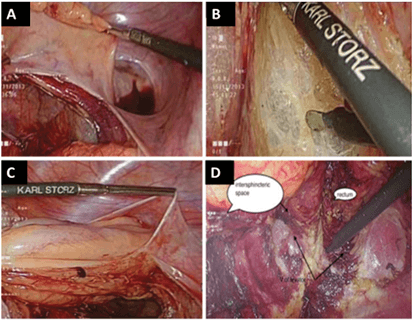
Figure 2: A) Total mesorectal excision (lateral dissection). B) Dissection in the posterior plane. C) Anterior dissection of the bladder. D) Identification of the intersphincteric plane from the abdominal side. Images used with permission from Pai et al. [18].
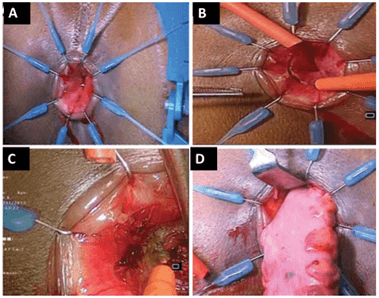
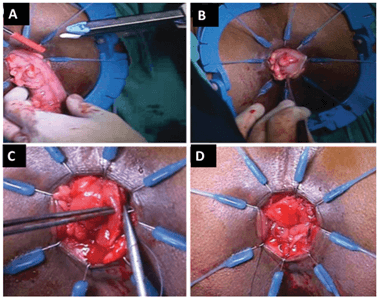
Depending on the position of the tumor, the cut is made at or below dentate line to enter the intersphincteric space, and dissection is continued until the cut joins the low abdominal dissection, as shown in (Figure 3). The rectal stump is then delivered through the anal opening and divided 10 cm above the viable proximal margin of the tumor, together with inferior mesenteric vessels and nodes. The resected DRM is examined by intra-operative frozen section to confirm R0 resection, as shown in (Figure 3). A straight CAA is then hand-sewn, and a protective diverting temporal ileostomy is done. Figure 4 shows a straight CAA. The abdomen is washed, and drains inserted, with the usual postoperative management done according to department protocol.
Figure 3: A) The Lone Star retractor applied for better exposure. B) Incision made in the rectal mucosa at the dentate line. C) Dissection in the intersphincteric plane until the abdominal part of the dissection is reached. D) Extraction of the specimen through the anus. Images used with permission from Pai et al. [18].
Figure 4: A) Dividing the colon with linear cutter. B) Aligning the remnant colon in the correct orientation. C) Handsewn coloanal anastomosis using vicryl 3/0 suture. D) Completed coloanal anastomosis. Images used with permission from Pai et al. [18].
Outcomes of ISR
I Morbidity and Mortality of ISR
ISR offers good functional anal sphincter and oncologic outcome results that afford acceptable anal continence, as an apportion to APR, for patients with very low rectal cancer [19-21]. However, like any other surgical procedure, it comes with possible morbidity and mortality that must be explained and consented to by the patient [19-21]. The decision to undergo ISR must be made by the patient after receiving information on short-term and long-term outcomes of ISR with morbidity and mortality [19-21]. Short-term outcomes include perioperative complications; long-term outcomes include oncologic outcomes, anal sphincter function outcomes that avoid stoma, QoL determined by levels of anal continence, and sexual and genitourinary complications [19-21]. Most patients prefer ISR to avoid a permanent stoma [15].
Shirouzu et al. reported an ISR overall morbidity of 7.5-38.3% and an operative mortality of 0-1.7% [15]. The common short-term outcomes included anastomotic leaks leading to pelvic abscess, colonic conduit ischaemia resulting in colonic stenosis and necrosis, and injury to adjacent organs that resulted in genitourinary dysfunction [8]. Anastomotic leakage was common at 4.3-48%, and colon conduit stenosis at 8.4-23.3%. These outcomes are not different from those of LAR but are lower than APR.
II Oncologic Outcomes of ISR
The primary goal of ISR is to obtain a safe R0 oncologic DRM comparable to LAR and APR, but that offers local disease control with minimal local recurrence, and disease-free survivor (DFS) and overall survival (OS) as with LAR and APR [19-21]. ISR has been reported to be technically feasible and offers oncologic outcomes comparable to curative resections such as APR and LAR [4]. Park et al. report a local recurrence of 4-13% in their series, following sphincter-preserving surgery for very low rectal cancer [14]. They state that involvement of the CRM, when the margin is less than 1mm (accepted as a radial negative margin), is a predictor of local recurrence and a determinant of survival after curative surgery for very low rectal cancer [14]. However, achieving a radial negative margin for very low rectal cancer is difficult as the mesorectum has less fat, and the external sphincter muscle is encountered instead of mesorectal fat [4]. Local recurrence is commonly reported at 2.6-32% [22]. Martin et al. reported a mean DRM of 17.1mm, CRM negative of 96%, a local recurrence of 6.7%, DFS of 78.6% and OS of 86.3% [23].
Even though most patients present at time of diagnosis with locally advanced disease in T4 stage and nodal disease, NACTCR affords a low local recurrence of 6%, avoidance of a positive CRM, downstaging and downsizing to increase the possibility of success in sphincter-preserving surgery, and avoidance of a permanent stoma, except where external sphincter involvement persists in poorly differentiated adenosarcomas [6, 9]. Rullier et al. reported a 2% recurrence rate in patients who received NACTCR for T3 tumors, with 5-year OS of 81% and DFS of 71%, after median follow-up of 40 months [24]. The Japanese routinely administer radiotherapy for tumors up to T3, even with node negative disease [24]. However, Shirouzu et al. report a higher surgical complication rate, poor anal function, and increased sexual and genital urinary complication rate with the use of neoadjuvant radiotherapy [8]. A good oncologic outcome is not based on DRM but on a negative CRM, improved by NACTCR after clinical assessment by digital rectal exam and MRI [8, 11].
III Functional Outcomes of ISR
Despite most patients achieving the objective of oncologic R0 resection (the loss of internal anal sphincter, rectal reservoir and radiation therapy (RT)), there are reports of ISR outcomes that have been shown to significantly contribute to an unsatisfactory anal continence outcome, impacting on QoL after ISR [8]. Pelvic dissection done by the TME nerve-sparing technique also comes with sexual dysfunction and genitourinary problems following both surgery and RT, due to injury to parasympathetic and sympathetic nerves of the hypogastric plexus nerves, even though the rates are better than APR [3, 6, 9]. Problems of continence, urgency, stool fragmentation, and soiling due to anal dysfunction, LAR syndrome, are experienced by most patients in the postoperative period and are an inevitable consequence of ISR. The aim of treatment with the ISR procedure is to minimize their occurrence [25].
Quentin et al. reports that the TME technique has improved the sexual and urinary functional complications, from 10-30% to 40-60% after conventional rectal surgery to 0-12% and 10-35% after TME surgery [6]. They also report that ISR had no impact on male sexual dysfunction and tumor characteristics, compared to partial or total TME done in pelvic exenteration procedures [6]. Martin et al. report a mean of 2.7 bowel movements per day as acceptable continence for 51.2% of their patients, while 29.1% had fecal soiling, 23.8% had flatus incontinence, and 18.1% had urgency [6, 23]. The Wexner score and Fecal Incontinence Severity Index (FISI) have been used to assess the sphincter function outcome, though such an assessment is subjective. Anal manometry-use is not practical in most institutions, including ours, and the scores have varied based on the type of CAA [11, 25]. Constantine et al. report that significant improvement is obtained after a colonic J-pouch anastomosis, compared to straight CAA, in the first year postoperatively, but function is not sustained 2 years after surgery [9]. However, J-pouch colonic anastomosis is difficult to construct because the colon has a bulky mesentery that can compromise either colon conduit perfusion in a narrow pelvis or inadequate length of colon conduit, or both [15]. Schiessel reported that good sphincter function is a consequence of good ISR technique [15]. The anal sphincter function must be assessed before closure of the colostomy, and if anal sphincter function is not adequate, anal training can be done before closure of colostomy to avoid continence problems [15]. Patient dissatisfaction after ISR and stoma requirement due to continence problems are rare [15].
Conclusion
ISR when done by TME principals achieves good oncologic outcomes while preserving the anal sphincter for very low rectal cancer, with acceptable anal sphincter function and QoL. The DRM has evolved to less than 1 cm with R0, and negative CRM resections with help of NACTCR and MRI imaging have resulted in improved ISR technique, with good outcomes of DFS and OS comparable to LAR and APR.
Conflicts of Interest
None.
Funding
None.
Acknowledgement
None.
Ethical Approval
Not applicable.
Article Info
Article Type
Review of the LiteraturePublication history
Received: Fri 05, Mar 2021Accepted: Tue 16, Mar 2021
Published: Sat 03, Apr 2021
Copyright
© 2023 Avanish Saklani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.04.03
Author Info
Seke Manase Ephraim KAZUMA Rigved Nittala Vivek Sukumar Mufaddal Kazi Avanish Saklani
Corresponding Author
Avanish SaklaniProfessor and Consultant Colorectal Surgeon and Head of Department of Colorectal Surgical Oncology, Tata Memorial Hospital, Parel, Mumbai, India
Figures & Tables
References
1. Cipe G, Muslumanoglu M, Yardimci E, Memmi N, Aysan E (2012) Intersphincteric resection and coloanal anastomosis in treatment of distal rectal cancer. Int J Surg Oncol 2012: 581258. [Crossref]
2. Arya S, Das D, Engineer R, Saklani A (2015) Imaging in rectal cancer with emphasis on local staging with MRI. Indian J Radiol Imaging 25: 148-161. [Crossref]
3. Zedan A, Tawfik A, Aboeleupn E, Salah A, Morsy A (2019) Intersphincteric resection is the optimal procedure for very low rectal cancer: techniques, morbidity, oncologic and functional outcomes. J Cancer Ther 10: 400-410.
4. Park IJ, Kim JC (2018) Intersphincteric resection for patients with low-lying rectal cancer: Oncological and functional outcomes. Ann Coloproctol 34: 167-174. [Crossref]
5. Collard M, Lefevre JH (2020) Ultimate functional preservation with intersphincteric resection for rectal cancer. Front Oncol 10: 297. [Crossref]
6. Denost Q, Rullier E (2017) Intersphincteric resection pushing the envelope for sphincter preservation. Clin Colon Rectal Surg 30: 368-376. [Crossref]
7. Hong KS, Moon N, Chung SS, Lee RA, Kim KH (2015) Oncologic outcomes in rectal cancer with close distal resection margins: a retrospective analysis. Ann Surg Treat Res 89: 23-29. [Crossref]
8. Shirouzu K, Murakami N, Akagi Y (2017) Intersphincteric resection for very low rectal cancer: a review of the updated literature. Ann Gastroenterol Surg 1: 24-32. [Crossref]
9. Spanos CP (2012) Intersphincteric resection for low rectal cancer: an overview. Int J Surg Oncol 2012: 241512. [Crossref]
10. Molnar C, Vlad Olimpiu B, Marian B, Cornelia T, Simona G (2018) Survival and functional and oncological outcomes following intersphincteric resection for low rectal cancer: short-term results. J Int Med Res 46: 1617-1625. [Crossref]
11. Kumar S, Rohila J, Kg D, Sasi SP, Desouza A et al. (2020) Functional outcomes of intersphincteric resection (ISR) for low rectal cancers : results from a tertiary cancer centre in India. Eur J Surg Oncol 46: e101.
12. Zbar AP (2007) Sir W Ernest Miles. Tech Colosproctol 11: 71-74. [Crossref]
13. Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1: 1479-1482. [Crossref]
14. Parks AG (1972) Transanal technique in low rectal anastomosis. Proc R Soc Med 65: 975-976. [Crossref]
15. Schiessel R (2012) Surgical technique of intersphincteric resection. In: Schiessel R., Metzger P. (eds) Intersphincteric Resection for Low Rectal Tumors. Springer, Vienna.
16. Guedj N, Maggiori L, Poté N, Norkowski E, Cros J et al. (2016) Distal mural and tumour spread in the mesorectum after neoadjuvant radiochemotherapy in rectal cancer: about 124 consecutive patients. Hum Path 52: 164-172. [Crossref]
17. National Comprehensive Cancer Network (2020) NCCN clinical practice guidelines in oncology: rectal cancer Version 6.2020.
18. Pai VD, Sugoor P, Patil PS, Ostwal V, Engineer R et al. (2017) Laparoscopic versus open approach for intersphincteric resection—results from a tertiary cancer center in India. Indian J Surg Oncol 8: 474-478. [Crossref]
19. Saito N, Ono M, Sugito M, Ito M, Mohiro M et al. (2004) Early results of intersphincteric resection for patients with very low rectal cancer: an active approach to avoida permanent colostomy. Dis Colon Rectum 47: 459-466. [Crossref]
20. Tiret E, Poupardin B, McNamara D, Dehni N, Parc R (2003) Ultra low anterior resection with intersphincteric dissection—what is the limit of safe sphincter preservation? Colorectal Dis 5: 454-457. [Crossref]
21. Vorobiev GI, Odaryuk TS, Tsarkov PV, Talakin AL, Rybakov EG (2004) Resection of rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultra low rectal carcinoma. Br J Surg 91: 1506-1512. [Crossref]
22. Saito N, Sugito M, Masaki I, Kabayashi A, Nishizawa Y et al. (2009) Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg 33: 1750-1756. [Crossref]
23. Martin ST, Heneghan HM, Winter DC (2012) Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg 99: 603-612. [Crossref]
24. Rullier E, Laurent C, Bretagnol F, Rullier A, Vandrely V et al. (2005) Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg 241: 465-469. [Crossref]
25. Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R et al. (2004) Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum 47: 832-838. [Crossref]
