Remote Ischemic Preconditioning on New Onset Post-Cardiac Surgery Atrial Fibrillation: A Single-Centre Prospective Clinical Study
A B S T R A C T
Background: Remote ischemic preconditioning (RIPC) has been shown to reduce myocardial ischemia-reperfusion injury. However, its efficacy in preventing postoperative atrial fibrillation (POAF) remains unsettled.
Methods: A total of 97 eligible patients were prospectively randomized to receive either RIPC or sham-RIPC (control) prior to coronary artery bypass graft (CABG) surgery. RIPC was performed by applying 3 alternating cycles of a 5-min upper limb ischemia and reperfusion using a blood-pressure cuff. The primary endpoint was the incidence of POAF. Secondary endpoints included cardiac troponin T (cTnT) and H2O2 serum concentration after revascularization, and P-wave duration (PWD) on a 12-lead electrocardiogram.
Results: Twelve out of 49 RIPC patients (24.5%) and 18/48 of control patients (37.5%) developed POAF (p=0.165, χ2-test). H2O2 levels were significantly increased 30 min after revascularization in both groups compared to pre-clamping values (8.8±6 vs 25.5±2 and 8.5±5 vs 39±15.5 µM/L in the RIPC and control group, respectively; P<.001, within-group analysis). However, mean differences of H2O2 levels after reperfusion were lower in RIPC patients than in controls (P<.05). cTnT concentrations though increased between 6 and 12 h after operation in both groups, they began to fall later only in the RIPC group. PWD became shorter in RIPC treated patients but not in controls when measured postoperatively (82±13 vs 75±11 ms, P<.01).
Conclusion: RIPC did not significantly reduce the incidence of POAF despite decreases in cTnT/H2O2 levels and PWD, indicating that not the extent of myocardial injury but the injury itself triggers the electrophysiologic mechanisms underlying the development of this arrhythmia.
Keywords
Cardiac surgery, postoperative atrial fibrillation, remote ischemic preconditioning, cardiac troponin T, oxidative stress
Introduction
Postoperative atrial fibrillation (POAF) is the most frequently occurring arrhythmia during the first 2-3 days following coronary artery bypass graft (CABG) surgery. POAF may worsen the clinical outcomes by increasing the risk of embolic stroke, exacerbating acute cardiac or renal failure, and thus, prolonging hospitalization and further increasing mortality risk [1, 2]. This arrhythmia has been attributed to several factors associated with the surgical intervention per se, as well as by ischemia-reperfusion injury caused after graft opening. In this context, although the electrophysiologic substrate predisposing to POAF still remains unknown, high oxidative stress and intracellular Ca2+ overload during early reperfusion may promote the development of early afterdepolarizations and arrhythmogenesis. Remote ischemic preconditioning (RIPC) has been proposed as a simple non-invasive procedure that limits ischemia-reperfusion myocardial injury by attenuating oxidative stress and mitochondrial dysfunction [3, 4]. Due to controversial reports existed on the efficacy of RIPC in preventing POAF after cardiac surgery, we conducted this study to reassess the incidence of this arrhythmia in remotely conditioned patients who underwent CABG surgery [5-7].
Material and Methods
We conducted a randomized, allocation-concealed, single-blind (outcome assesors), single-site clinical trial. A total of 97 patients were evaluated between April 2017, and October 2018 for the incidence of POAF after elective on-pump CABG surgery. Following the approval of the study by the university hospital’s ethics committee, all patients were informed about their participation and signed the consent form. Participants were prospectively randomized to receive RIPC or no RIPC (control). Exclusion criteria were type II diabetes as well as history of AF or other cardiac arrhythmias requiring antiarrhythmic therapy, preoperative myocardial infarction, cardiogenic shock, or emergent CABG and/or valve surgery. Anesthesia was induced with midazolam, fentanyl, etomidate and pancuronium in a patient-adapted dosage regimen. Maintenance of deep anesthesia was achieved by sevoflurane volatile gas, propofol, and remifentanyl administered with a pump. After induction of anesthesia and before skin incision, RIPC was performed by applying 3 consecutive cycles of 5 min blood-pressure cuff inflation to 200 mmHg to the upper left arm, followed by 5 min reperfusion while the cuff was deflated. In the control group, the cuff was placed around the arm but not inflated. The primary endpoint was the incidence of POAF in both groups occurring after 2-3 days postoperatively or until discharge. For this purpose, patients were equipped with a 24-h Holter ECG device (custo flash 200, Ottobrunn, Germany); basic ECG variables were determined by 12-lead standard ECGs at 50 mm paper speed. Myocardial ischemia/reperfusion injury was estimated by measuring cardiac troponin T (cTnT) concentrations. For further exploration of the cardioprotective mechanisms of RICP, in a subgroup of patients (N=25) oxidative stress was evaluated by measuring serum H2O2 levels and total antioxidant capacity (TAC).
For H2O2 evaluation, blood samples were obtained via a catheter from the femoral artery before aortic clamping as well as 30 min and 2 h after revascularization. Determination of H2O2 was performed according to the procedure described by Franz et al. [8]. Briefly, standard solutions of H2O2 (0-1000 μM) were prepared. 10 μL of standards and samples were incubated with 200 μL of the reaction mixture consisting of HRP (25 mU), TMB and substrate solution at a ratio of 1:10:100 in exposed plates. After 15±5 min incubation, the reaction was quenched by addition of 2N HCl solution, and the absorbance was determined at 450 nm (reference: 620 or 570 nm). Serum peroxide levels were calculated relative to the absorbance in the peroxide standard curve; the results were expressed in μM of H2O2. In addition, TAC was examined using the 1,1-diphenyl-2-picrylhydrazil (DPPH). TAC reflects the capability of the plasma components to scavenge reactive species and has been used as an indicator of the overall antioxidant capacity of plasma. In the presence of hydrogen donors existing in the plasma, the free radical DPPH* is reduced to the corresponding hydrazine. The depletion of the radical is evaluated by the decrease in absorbance at 520 nm (% absorbance reduction= (Abs blank-Abs sample)/Abs blank x100 µM DPPH scavenged/ml= [(%Abs reduction/100) x50x50]/1000). Pre- and post-operatively, the duration of extracorporeal circulation, aortic cross-clamp time, LV ejection fraction (2D-ECHO), and biochemical laboratory findings were additionally determined. Basic ECG variables such as P-wave duration (PWD), PQ, QRS, QT, QTc (QT corrected for heart rate according to Bazett's formula), and Tpeak-Tend (Tp-Te) intervals were determined pre- and post-operatively in the intensive care unit (ICU) immediately after stabilization of sinus rhythm. Tp-Te indicates the extent of transmural dispersion of ventricular repolarization as a measure of ventricular arrhythmogenicity.
Statistical Analysis
For categorical variables, descriptive statistics are summarized as frequency (%) and comparisons between the RIPC and control group were performed with a χ2 test. For continuous variables (expressed as mean±SD) differences between groups were examined with the Student's t test for independent samples. or the Mann Whitney test as appropriate (Kolmogorov-Smirnov Normality Test). Data were analyzed using GraphPad Prism 5.01 (GraphPad Software, San Diego, CA, USA). A p value less than 0.05 was considered as statistically significant. In case of multiple comparisons, t-test were followed by a Bonferroni adjustment.
Figure 1: Serum cTnT levels in patients subjected to RIPC vs not treated before the surgical procedure. Troponin concentrations increased significantly between 6- and 12-hours post-surgery but decreased thereafter solely in the RIPC patients. **P<.01, ***P<.001.
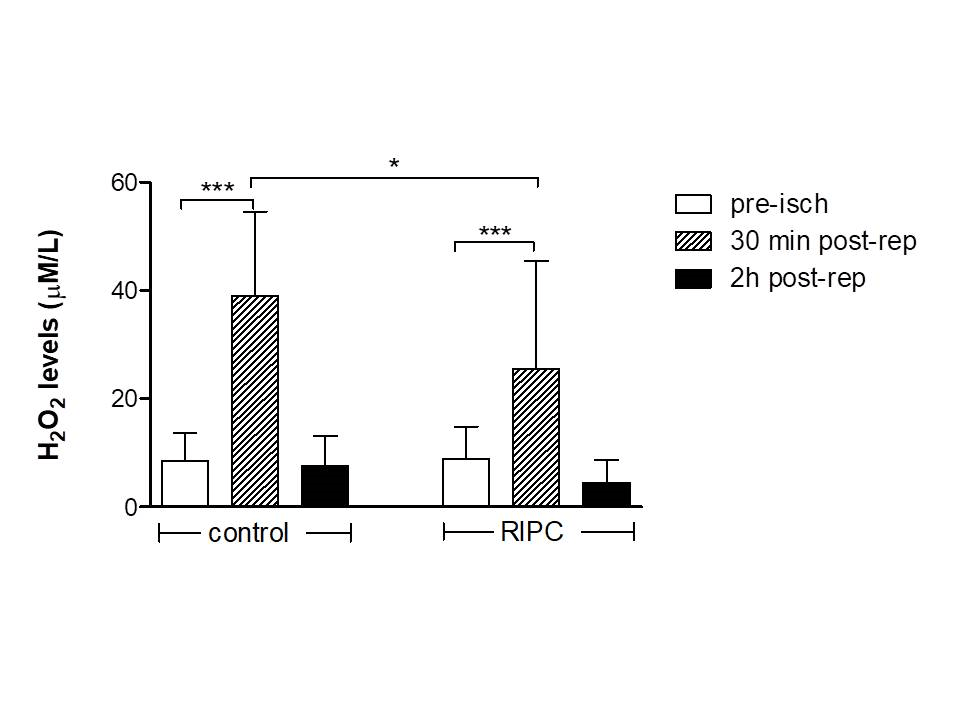
Figure 2: Levels of H2O2 increased 30 min post-reperfusion (post-rep) compared to pre-ischemia (pre-isch) values in both groups of patients, but two hours later they recover to pre-isch levels. Notably, the increases of oxidative stress were lower in the RIPC group. *P<.05, ***P<.001.
Results
Patients were randomized on a 1:1 basis. No stratification factors were used, and no block randomization was applied. On the day of surgery, patients were assigned to undergo either RIPC or no RIPC (control). Demographic as well as baseline and intraoperative characteristics are presented on Table 1. Left ventricular ejection fraction (LVEF) and left atrial diameter were not significantly different between groups. Total cardiopulmonary bypass (CPB) time was similar in the two groups, while cross-clamp time was longer in the RIPC group (P<0.05). POAF, albeit less common in RIPC patients, remained relatively high (24.5%) when compared to the control group (37.5%) (Table 2). Pre-operatively, PWD was similar between the two groups; however, post-operatively, PWD became shorter only in patients belonging to the RIPC group (P<0.01, post- vs pre-op values).
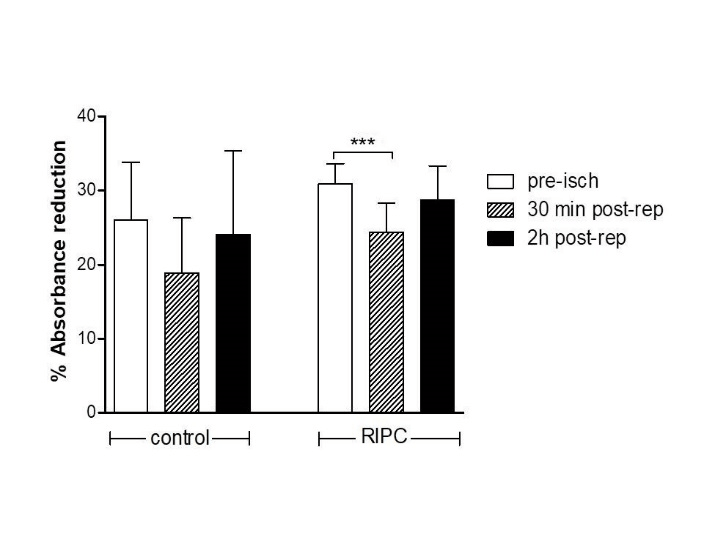
Preoperative cTnT serum concentrations were within normal limits (<50 ng/L). As expected, serum troponin concentrations significantly increased up to 12 h after operation in both groups, whereas they started and continued to fall between 12 and 72 h post-surgery only in the RIPC group (Figure 1). CRP was significantly elevated after surgery in both groups, while urea levels decreased postoperatively, albeit not significantly, only in the RIPC group, compared to the corresponding values in the control group (Table 2). Myocardial ischemia followed by aortic declamping-induced reperfusion resulted in a pronounced increase in H2O2 serum concentrations in both groups, compared to the pre-ischemia levels (Figure 2, P<.001). Thirty minutes post-reperfusion H2O2 levels were significantly higher in the control (39±15.6 µM/L, N=13) vs RIPC (25.5±19.9 µM/L, N=20) group (P= 0.047). Two hours after revascularization, H2O2 concentrations recovered to the pre-clamping values. In the control group (N=15), total antioxidant capacity (TAC) measured using the %absorbance reduction, was decreased from 26±7.8% during pre-clamping to 18.9±7.4% 30 min post reperfusion (P=.051), and then progressively increased to 24.1±11.2% 2 h post reperfusion (P=.66 vs pre-ischemia levels) (Figure 3). In the RIPC group (N=10), TAC pre-clamping levels decreased from 30.9±2.7 to 24.4±3.9% 30 min post reperfusion (P<.001), and then progressively increased to 28.7±4.6% 2 h after revascularization (P=.121 vs pre-ischemia values).
Figure 3: Total antioxidant capacity (TAC) estimated by means of %Absorbance reduction as a function of plasma scavenger depletion in control and RIPC patients. Scavenger capacity 30 min post-reperfusion (post-rep) was relative higher in the RIPC group than in the control group (P=.042), despite similar reductions when compared to the pre-ischemia (pre-isch) values. *P<.05, ***P<.001.
Table 1: Baseline and clinical data.
|
|
Control (N=48) |
RIPC (N=49) |
|
Demographics and comorbidities |
|
|
|
Age (years) |
63.9±9.1 |
65.4±8.5 |
|
Sex (male) |
39 (81%) |
42 (86%) |
|
Arterial hypertension |
30 (62.5%) |
27 (55%) |
|
Preoperative medication |
|
|
|
β-blockers |
29 (60%) |
37 (75.5%) |
|
Digoxin |
0 |
3 |
|
ACE inhibitors/ARBs |
20 (42%) |
18 (37%) |
|
Eplerenone |
2 |
11 |
|
Spironolactone |
0 |
2 |
|
Echocardiography |
|
|
|
LVEF (%) |
44.2±10.6 |
47.86±11 |
|
Left atrial diameter (mm) |
38.6±3.7 |
39.7±5.8 |
|
Intraoperative data |
|
|
|
CABG x II |
3 (6.3%) |
5 (10.2%) |
|
CABG x III |
33 (68.7%) |
35 (71.5%) |
|
CABG x IV |
12 (25%) |
9 (18.3%) |
|
Valve replacement+CABG |
2 |
3 |
|
Aortic cross-clamp time (min) |
63.7±25 |
68.58±18.5* |
|
Total CPB time (min) |
98.1±28.1 |
102.4±28.4 |
ACE, angiotensin conveting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; II, III, IV indicate the number of grafts; CPB, cardio-pulmonary bypass. *P<0.05 (control vs RIPC).
Table 2: Comparison of postoperatively measured biochemical parameters, incidence of postoperative atrial fibrillation (POAF), and evaluation of basic ECG variables during sinus rhythm.
|
|
CONTROL (N=48) |
RIPC (N=49) |
||
|
|
Pre-Op |
Post-Op |
Pre-Op |
Post-Op |
|
K+ (mmol/L) |
4.4±0.5 |
4.5±0.4 |
4.4±0.6 |
4.3±0.5 |
|
Creatinine (mg/dL) |
1.107±0.2 |
1.148±0.3 |
1.059±0.3 |
1.078±0.3 |
|
Urea (mg/dL) |
45±18 |
47±24 |
41±14 |
38±20 |
|
CRP (mg/dL) |
0.46±0.3 |
1.2±0.4*** |
0.33±0.2 |
0.8±0.1*** |
|
POAF (%) |
18 patients (37.5%) |
12 patients (24.5%) |
||
|
PWD (ms) |
83±12 |
86±19 |
82±13 |
75±11** |
|
P-Q (ms) |
150±28 |
150±37 |
149±24 |
150±26 |
|
QRS (ms) |
104±22 |
107±25 |
86±17 |
87±18 |
|
QT (ms) |
396±35 |
392±42 |
383±46 |
362±28**** |
|
QTc (ms) |
405±42 |
430±52**** |
392±41 |
398±32 |
|
Tp-Te (ng/L) |
77±14 |
80±18 |
74±14 |
72±14 |
Values are expressed in ms (mean±SD). *P<0.05, **P<0.01, ***P<0.001 postoperative (post-op) vs preoperative (pre-op) within the control or RIPC group. N= number of patients
Discussion
The major finding of this study was that patients undergoing RIPC did not show a significantly lower incidence of POAF, compared to nonconditioned patients. Reduced oxidative stress, cTnT levels, and shorter PWD in conditioned patients, seemingly were unable to exert a valuable arrhythmia-preventing effect. POAF has clinically been established as a spontaneously self-terminated arrhythmia, which however may increase the risk of early complications after cardiac surgery. Although this arrhythmia appears during the initial postoperative period, it can trigger a cascade of molecular mechanisms inducing electrical remodeling and persistency of arrhythmia even in later stages after bypass operation [9, 10]. For this reason, many efforts have been undertaken to prevent or convert it especially in patients with compromised left ventricular function. RIPC has been proposed as a simple, easily implemented, and costless noninvasive intervention limiting myocardial ischemia-reperfusion injury via complex cellular pathways, thereby enhancing cell survival [11-13]. Although RIPC has been shown to reduce myocardial infarct size, its efficacy against POAF is still questionable [14]. Slagvold et al. observed 36% lower incidence of POAF in RIPC patients with no differences in plasma concentrations of cTnT or CK-MB between treated and nontreated groups [7].
Furthermore, a reduction in the incidence of POAF after RIPC has been achieved even in patients undergoing CABG surgery using the anti-preconditioning anesthetic drug propofol [15]. In fact, these findings limit the impact of ischemia-reperfusion injury and the role of intraoperatively given anesthetics in the pathogenesis of POAF occurring several days after the operative procedure. Increased oxidative stress after myocardial ischemia and particularly upon reperfusion, has been reported to promote electrophysiologic remodeling associated with increased late sodium current and intracellular Ca++ gradients, which could be potentially arrhythmogenic through the formation of early or late afterdepolarizations [16]. This partially explains why treatment with ranolazine, a late sodium channel blocker, markedly reduced the incidence of POAF after CABG surgery [17]. The fact that a substantial number of post-cardiac surgery patients develop AF but not ventricular tachyarrhythmias would link to mechanisms arising from an intraoperatively mediated atrial injury. In this line, however, although controversial results exist regarding the incidence of POAF between on-pump and off-pump coronary bypass surgeries, a number of studies suggest that patients undergoing off-pump bypass surgery also often developed POAF [18]. In addition, given that all patients undergoing CABG surgery were subjected to almost the same interventions, including similar cross-clamp times and procedure-related atrial traumatization, but only approximately 1/3 of those showed POAF, it can be assumed that the operative procedure per se is unlikely to be the main responsible factor for the appearance of this arrhythmia. Therefore, it is reasonable to consider that other factors, independent from the surgical procedure, are prevailingly involved in POAF.
In this respect, it seems possible that not the size of reperfusion injury but the injury itself is responsible for the appearance of LV diastolic dysfunction, that presumably results in elevating left atrial pressures and atrial wall stretch [19]. The latter has been shown to shorten atrial action potential duration and refractoriness, thereby predisposing to reentry [20, 21]. On the other hand, increased late sodium current and alterations in mitochondrial membrane potential due to ischemia-reperfusion induced oxidative stress can result in elevation of intracellular Ca++, generating early afterdepolarizations triggered activity which may also be conducive to reentrant excitability [22]. Collectively, the effects of RIPC on gating of atrial cell membrane channels promoting abnormal automaticity and abbreviation of refractoriness after cardiac surgery remain obscure. Notwithstanding, the observation that ranolazine given preoperatively during sinus rhythm prevented POAF suggests that the blockade of late sodium plays an important role in the initiation of this arrhythmia [17]. Moreover, an additional use-dependent atrial-selective blockade of fast sodium channels with ranolazine that possibly prolonged post-repolarization refractoriness during AF, may unmask also reentry involvement in POAF [23].
Furthermore, the effects of preconditioning on gap-junctional conduction delay in ischemic atrial muscle has also been considered and could further clarify the relationship between preconditioning, conduction, and atrial arrhythmogenesis after surgery. Accordingly, Zhu et al. demonstrated a less extensive gap-junctional conduction delay in preconditioned ischemic ventricle [24]. This might be attributed to a decreased cytosolic Ca++ accumulation as a result of preconditioning [22, 25]. In clinical settings, Passman et al. used PWD >110 ms as a measure of atrial conduction delay to identify patients being at higher risk for the development of POAF [26].
Limitations of Study
The lack of evaluating previous poarrhythmic substrate that possibly increased the vulnerability to POAF in patients of the two groups, obviously limited the efficacy of remote ischemic preconditioning. Furthermore, a possible interference of propofol with preconditioning cannot be excluded, though propofol was used in all patients. Despite the relatively small number of patients in the study, as well as the limited number of blood samples for assessing oxidative stress and total antioxidant capacity, our findings demonstrated clear differences between patients receiving or not receiving RIPC treatment.
Conclusion
Despite the limitations of this study, our findings show that preconditioning mediated myocardial protection after ischemia and reperfusion, does not necessarily means arrhythmia protection. The present findings confirm data stemming from other studies demonstrating the inefficacy of RIPC in reducing episodes of POAF after cardiac surgeries. Most studies reported an incidence of POAF >25% of patients receiving RIPC, a percentage close to that observed in non-RIPC patients. Reduced serum cTnT and oxidative stress after coronary revascularization surgery did not help explain distinct electrophysiologic alterations in atria that could facilitate the occurrence of this arrhythmia. Experimental research is necessary to detect the time course of such alterations, e.g., atrial propagation time, refractory period, and early or delayed afterdepolarizations, during the first critical postoperative days at which POAF often occurs.
Conflict of interest
We declare that we have no conflicts of interest.
Acknowledgements
We thank Malavaki C, MSc, PhD for evaluating serum TAC levels.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Article Info
Article Type
Research ArticlePublication history
Received: Fri 08, Nov 2019Accepted: Mon 25, Nov 2019
Published: Sat 14, Dec 2019
Copyright
© 2023 Aidonidis Isaac. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2019.04.09
Author Info
Aidonidis Isaac Befani Christina Dipla Konstantina Hatziefthimiou Apostolia Liakos Panagiotis Papadopoulos Eleftherios Simopoulos Vassilios Stamoulis Konstantinos Tsilimingas Nikolaos
Corresponding Author
Aidonidis IsaacDepartment of Physiology, of Larissa Medical School, University of Thessaly, Larissa, Greece
Figures & Tables
Table 1: Baseline and clinical data.
|
|
Control (N=48) |
RIPC (N=49) |
|
Demographics and comorbidities |
|
|
|
Age (years) |
63.9±9.1 |
65.4±8.5 |
|
Sex (male) |
39 (81%) |
42 (86%) |
|
Arterial hypertension |
30 (62.5%) |
27 (55%) |
|
Preoperative medication |
|
|
|
β-blockers |
29 (60%) |
37 (75.5%) |
|
Digoxin |
0 |
3 |
|
ACE inhibitors/ARBs |
20 (42%) |
18 (37%) |
|
Eplerenone |
2 |
11 |
|
Spironolactone |
0 |
2 |
|
Echocardiography |
|
|
|
LVEF (%) |
44.2±10.6 |
47.86±11 |
|
Left atrial diameter (mm) |
38.6±3.7 |
39.7±5.8 |
|
Intraoperative data |
|
|
|
CABG x II |
3 (6.3%) |
5 (10.2%) |
|
CABG x III |
33 (68.7%) |
35 (71.5%) |
|
CABG x IV |
12 (25%) |
9 (18.3%) |
|
Valve replacement+CABG |
2 |
3 |
|
Aortic cross-clamp time (min) |
63.7±25 |
68.58±18.5* |
|
Total CPB time (min) |
98.1±28.1 |
102.4±28.4 |
ACE, angiotensin conveting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; II, III, IV indicate the number of grafts; CPB, cardio-pulmonary bypass. *P<0.05 (control vs RIPC).
Table 2: Comparison of postoperatively measured biochemical parameters, incidence of postoperative atrial fibrillation (POAF), and evaluation of basic ECG variables during sinus rhythm.
|
|
CONTROL (N=48) |
RIPC (N=49) |
||
|
|
Pre-Op |
Post-Op |
Pre-Op |
Post-Op |
|
K+ (mmol/L) |
4.4±0.5 |
4.5±0.4 |
4.4±0.6 |
4.3±0.5 |
|
Creatinine (mg/dL) |
1.107±0.2 |
1.148±0.3 |
1.059±0.3 |
1.078±0.3 |
|
Urea (mg/dL) |
45±18 |
47±24 |
41±14 |
38±20 |
|
CRP (mg/dL) |
0.46±0.3 |
1.2±0.4*** |
0.33±0.2 |
0.8±0.1*** |
|
POAF (%) |
18 patients (37.5%) |
12 patients (24.5%) |
||
|
PWD (ms) |
83±12 |
86±19 |
82±13 |
75±11** |
|
P-Q (ms) |
150±28 |
150±37 |
149±24 |
150±26 |
|
QRS (ms) |
104±22 |
107±25 |
86±17 |
87±18 |
|
QT (ms) |
396±35 |
392±42 |
383±46 |
362±28**** |
|
QTc (ms) |
405±42 |
430±52**** |
392±41 |
398±32 |
|
Tp-Te (ng/L) |
77±14 |
80±18 |
74±14 |
72±14 |
Values are expressed in ms (mean±SD). *P<0.05, **P<0.01, ***P<0.001 postoperative (post-op) vs preoperative (pre-op) within the control or RIPC group. N= number of patients

References
- LaPar DJ, Speyer AM, Crosby IK, Fonner E Jr, Brown M et al. (2014) Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 98: 527-533. [Crossref]
- Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA et al. (2014) Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery. Clin Cardiol 37: 7-13. [Crossref]
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M et al. (2007) Effect of remote ischemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet 370: 575-579. [Crossref]
- Venugopal V, Ludman A, Yellon DM, Hausenloy DJ (2009) 'Conditioning' the heart during surgery. Eur J Cardiothorac Surg 35: 977-987. [Crossref]
- Lotfi AS, Eftekhari H, Atreya AR, Kashikar A, Sivalingam SK et al. (2016) Randomized controlled trial of remote ischemic preconditioning and atrial fibrillation in patients undergoing cardiac surgery. World J Cardiol 8: 615-622. [Crossref]
- Krogstad LE, Slagsvold KH, Wahba A (2015) Remote ischemic preconditioning and incidence of postoperative atrial fibrillation. Scand Cardiovasc J 49: 117-122. [Crossref]
- Slagsvold KH, Rognmo O, Høydal M, Wisløff U, Wahba A (2014) Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circ Res 114: 851-859. [Crossref]
- Franz T, Sirid G, Willibald W, Rudolf W (2003) Dual method for the determination of peroxidase-activity and total peroxides-iodide leads to a significant increase of peroxidase-activity in human sera. Anal Biochem 316: 147-153. [Crossref]
- Zhang L, Po SS, Wang H, Scherlag BJ, Li H et al. (2015) Autonomic remodeling: how atrial fibrillation begets atrial fibrillation in the first 24 hours. J Cardiovasc Pharmacol 66: 307-315.
- Lee SH, Kang DR, Uhm JS, Shim J, Sung JH et al. (2014) New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am Heart J 167: 593-600. [Crossref]
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR et al. (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106: 2881-2883. [Crossref]
- Hausenloy DJ, Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79: 377-386. [Crossref]
- Marczak J, Nowicki R, Kulbacka J, Saczko J (2012) Is remote ischaemic preconditioning of benefit to patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg 14: 634-639. [Crossref]
- Candilio L, Malik A, Barnard M, Ariti C, DiSalvo C et al. (2015) Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomized controlled clinical trial. Heart 101: 185-192. [Crossref]
- Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H et al. (2012) Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand 56: 30-38. [Crossref]
- Karagueuzian HS, Nguyen TP, Qu Z, Weiss JN (2013) Oxidative stress, fibrosis, and early afterdepolarization-mediated cardiac arrhythmias. Front Physiol 4: 19. [Crossref]
- Tagarakis G, Aidonidis I, Daskalopoulou S, Simopoulos V, Liouras V et al. (2013) Effect of ranolazine in preventing postoperative atrial fibrillation in patients undergoing coronary revascularization surgery. Curr Vasc Pharmacol 11: 988-991. [Crossref]
- Athanasiou T, Aziz O, Mangoush O, Al-Ruzzeh S, Nair S et al. (2004) Does off-pump coronary artery bypass reduce the incidence of post-operative atrial fibrillation? A question revisited. Eur J Cardiothorac Surg 26: 701-710. [Crossref]
- Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM et al. (2011) Diastolic dysfunction in patients undergoing cardiac surgery: a pathophysiological mechanism underlying the initiation of new-onset post-operative atrial fibrillation. J Am Coll Cardiol 58: 953-961. [Crossref]
- Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA et al. (2009) Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF. Heart Rhythm 6: 445-451. [Crossref]
- Babuty D, Lab M (2001) Heterogeneous changes of monophasic action potential induced by sustained stretch in atrium. J Cardiovascular Electrophysiology 12: 323-329. [Crossref]
- Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T et al. (2011) Reactive oxygen species-activated Ca/Calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res 108: 555-565. [Crossref]
- Simopoulos V, Tagarakis GI, Daskalopoulou SS, Daskalopoulos ME, Lenos A et al. (2014) Ranolazine enhances the antiarrhythmic activity of amiodarone by accelerating conversion of new-onset atrial fibrillation after cardiac surgery. Angiology 65: 294-297. [Crossref]
- Zhu J, Ferrier GR (1998) Ischemic preconditioning: antiarrhythmic effects and electrophysiological mechanisms in isolated ventricle. Am J Physiol 274: H66- 75. [Crossref]
- Steenbergen C, Perlman ME, London RE, Murphy E (1993) Mechanism of preconditioning: ionic alterations. Circ Res 72: 112-125. [Crossref]
- Passman R, Beshai J, Pavri B, Kimmel S (2001) Predicting post-coronary bypass surgery atrial arrhythmias from the preoperative electrocardiogram. Am Heart J 142: 806-810. [Crossref]
