Protective Effects of the Leaf Aqueous Extract of Manihot Esculenta Crantz Against High-Calorie Diet Combined with Alcohol and Sucrose Ingestion Induced Hypertension in Rats
A B S T R A C T
Manihot esculenta commonly known as cassava is widely distributed across the world. In sub-Saharan Africa, its leaves and tubers are commonly eaten by local populations, who often lend this plant the properties of a traditional medicine. The present study was aimed to evaluate the protective effects of the leaf aqueous extract of Manihot esculenta Crantz against high-calorie diet combined with alcohol and sucrose ingestion induced hypertension in rats. Male wistar rats were randomly divided into three groups of six rats each. Antihypertension activity was evaluated by daily oral administration of Manihot esculenta aqueous extract at the dose of 200 mg/kg bw to rats exposed to high fat/high sucrose diet and daily oral ingestion of sucrose (15%, 5 mL/kg) and ethanol (40°, 5 mL/kg). At the same time, the group 2 or positive control group received high fat/high sucrose diet and daily oral ingestion of sucrose (15%, 5 mL/kg) and ethanol (40°, 5 mL/kg). The last group received standard diet and distilled water (5 mL/kg, orally). During the experimental period (8 weeks), the body weight of animals was daily recorded while the glycaemia was measured every two weeks. The hemodynamic (blood pressure and heart beat) and some serum markers of lipid profile (Triglycerides, total cholesterol, LDL cholesterol and HDL cholesterol), endothelial (NO) and liver functions (AST and ALT) as well as oxidative stress parameters (SOD, CAT, GSH, MDA and TBARS) in the serum and aorta were measured at the end of the study.
Our results indicated that Manihot esculenta aqueous extract significantly reduced the body weight gain, diastolic blood pressure, aorta diameter, glycaemia, of animals submitted to both oral administration of sucrose, ethanol and high fat fat/high sucrose diet, and plant extract. Moreover, the administration of Manihot esculenta extract improved the vasodilatation by increasing the endothelial NO secretion. Furthermore, the treatment with this extract improved the balance of the oxidative stress markers and the level of triglyceride, cholesterol and transaminases.
Thereby, these results suggest that the leaf aqueous extract of Manihot esculenta could have protective effects against hypertension induced by high-calorie diet combined with alcohol and sucrose ingestion in rats.
Keywords
High fat/sucrose diet, endothelium, hypertension, Manihot esculenta, oxidative stress
Introduction
Associated with nearly a third of recorded deaths, cardiovascular disease (CVD) remains the leading cause of death worldwide today [1]. 9 million deaths per year worldwide are due to increased blood pressure, more than 10% of the annual total [2]. According to the WHO, it is diagnosed in front of a patient with systolic and diastolic pressure greater than 140/90 mmHg regular and/or after medication [3]. Also coronary heart disease, ischaemia, myocardial infarction and cardiovascular events are strongly correlated with increased blood pressure [4]. Pathologies and their complications which, in some cases, are preventable.
There are many factors that can hinder the activity of the endothelium, and therefore increase the risk of developing cardiovascular disease and/or stroke. The factors most often cited are obesity and the associated inflammatory state, through its ability to induce a low-grade inflammatory state, increase the recruitment of immune cells to the identified inflammatory site and the formation of a plaque. atheroma [5]. But also, diabetes mellitus, oxidative stress, through the increase in the production of free radicals such as the products of protein glycation, altered molecules capable of accelerate the formation of atheromatous plaque or ruptured aneurysm [6, 7].
There is no doubt that quality medical care makes it possible to maintain blood pressure at suitable values, significantly reducing the occurrence of complications and a priori cardiovascular mortality and morbidity [8]. However, while patient monitoring and access to quality treatment seems obvious in western countries, it is not always the case in black African countries. Many epidemiological studies have established an inverse correlation between cardiovascular morbidity and mortality and the consumption of products rich in polyphenols such as fruits, vegetables, red wine, cocoa and tea, to name a few [9]. According to most of these studies, the beneficial effect of polyphenols on cardiovascular health results in part from their direct effect on blood vessels and more specifically on the endothelium [10].
Manihot esculenta commonly known as cassava or tapioca is a plant of the Euphorbiaceae family cultivated in sub-Saharan Africa, Asia and America. The roots of this plant are a major source of carbohydrates, while the leaves are consumed as vegetables. They are rich in vitamins A and C, proteins and minerals, particularly Mg, Zn, Fe and Mn. Also, the leaves are rich in phytochemicals, which are valuable as natural antioxidants, and their consumption has been linked to reducing the risks of cancer, hypertension, diabetes, obesity and heart diseases [11]. Therefore, this study was designed to evaluate the protective effects of the leaf aqueous extract of Manihot esculenta Crantz against high-calorie diet combined with alcohol and sucrose ingestion induced hypertension in rats.
Materials and Methods
I Chemical and Instruments
Different reagents such as kits of AST, ALT, total cholesterol, triglyceride, HDL-cholesterol and LDL-cholesterol) levels used in this study, were purchased from Merck (Germany). Thiobarbituric acid (TBA), sulfuric acid, sodium carbonate (Na2CO3); sodium hydroxide (NaOH); sodium nitrite (NaNO2); disodium hydrogen phosphate (Na2HPO4), hydrogen peroxide (H2O2), copper sulphate; adrenalin, hexane (Hex), catalase (CAT), amylase, glutathione (GSH) reagents were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). Some drugs like diazepam and ketamine and solvents such as ethanol (95%) were purchase at the locale pharmacy. An accucheck active glucometer has being used to measure fasting blood glucose levels. A Vanguard brand V6500 Iohazard micro-centrifuge was used to separate the constituents of a mixture.
II Animals
Young male wistar rats, aged 10 to 12 weeks, were acquired from the Animal Physiology Laboratory of the University of Yaoundé I (Cameroon). All animal experiments were conducted in accordance with the ethical guidelines for laboratory animal use and care as described in the European Community Guidelines 2010/63/EU. The practices carried out on animals and the related protocols have been approved by the animal protection and use committee of the institutional ethics committee of the Cameroonian Ministry of Scientific Research and Technological Innovation.
III Plant Material Extract
The leavers of Manihot esculenta were bought on January 2021 at the market in Mfoundi Yaoundé, a town in the central region (Cameroon). Authentic samples have been identified at the National Herbarium of Cameroon by comparison with Betti J. L material number 261, from the Herbarium Collection Specimen Number 66276 HNC. After cleaning, the cucumbers were then air dried at ambient temperature and crushed into powder using a blender. This powder was dissolved in the extraction solvent (1 g of ground material per 30 ml of water) and allowed to macerate for 24 h at room temperature. The leaf aqueous extract of Manihot esculenta leaves (ME) obtained was used for the determination of the bioactive compounds of interest.
IV Determination of Total Polyphenols
The total phenolic content was quantified using the Folin-Ciocalteu (FC) reagent according to the method of Singleton and Rossi [12]. The aqueous extract of Manihot esculenta (30 µL) was mixed with 1 mL of FC reagent (previously diluted 10 times with distilled water). The mixture was incubated for 5 minutes at room temperature, then 0.7 mL of Na2CO3 solution (7%) was added. A second-long incubation period of 30 minutes was then carried out, the tubes were stored at room temperature and protected from light. The absorbance was determined at 750 nm. The polyphenol content was being quantified through the use of a standard catechin curve.
V Animals and Treatments
Throughout this experiment, twenty-four (24) rats of wistar strain weighing between 220 and 240 g were divided into three groups of six rats each. These animals were conditioned in the appropriate cages and maintained in a controlled environment under a cycle of 12 hours, in the light and 12 hours in the dark at 50% humidity. This acclimatization phase lasted a week during which they received standard diet (Table 1) and water ad libitum. After this period, rats are treated during eight weeks, in according to Bilanda et al. as follow [13]:
Table 1: Composition of the
experimental diets (g/kg diet) [48].
|
Ingredients |
Standard died (SD) |
High fat/High sucrose died
(HFHS) |
|
Milk
|
/ |
145 g |
|
Sucrose |
/ |
290 g |
|
Corn
starch of blew |
216 g |
188 g |
|
Corn
starch of maze |
324 g |
237 g |
|
Margarine
|
/ |
143,3 g |
|
Palm
oil |
50 g |
55 g |
|
Cellulose
powder |
/ |
30 g |
|
Mineral
mixture |
20 g |
20 g |
|
Vitamin
mixture |
5 g |
5 g |
Group 1 received a standard diet and distilled water (5 mL/kg of body weight by oral gavage).
Group 2 received high fat / high sucrose diet, ethanol 40° (5 mL/kg of body weight by oral gavage) and sucrose 15% (5 mL/kg of body weight by oral gavage).
Group 3 received high fat / high sucrose diet, associated to oral administration of ethanol 40° and sucrose 15% as previously mentioned, and the plant extract (200 mg/kg bw.) respectively.
During the experimental period, the body weight of each rat was daily recorded while glycaemia was assessed at the start, every two weeks and at the end of the study.
i Assessment of Physiological Parameters
At the end of the eight weeks of treatment, the animals of the different groups were anaesthetized by intraperitoneal injection of 15% urethane at a dose of 1.5 g/kg bw. Each anaesthetized animal was fixed in the supine position on a cork board. The penis has been bared. After which a first ligature was placed under the dark vein on the ventral side. An incision of the vein was made and a catheter filled with 10% heparinized Mac Even was introduced into the vein and maintained by a second ligature.
The direct method was used for assessment of blood pressure. It consists of recording the blood pressure using a catheter introduced into the carotid artery of anaesthetized rat. To make an incision was made at the neck, the stern hyoid muscles and the trachea were removed. The carotids have been gently separated from the nerve fibers. A cephalic ligation was performed and a waiting wire placed under the artery. Then a vascular clamp was placed as low as possible towards the heart near the lead wire. An incision was made between the first ligature and the waiting wire. The free tip of the catheter connected to the transducer was introduced into the carotid artery towards the heart and held by the second ligature. Blood pressure and heart rate were recorded after opening the valve that connects the carotid artery to the transducer. When the clamp is removed, arterial blood flows through the catheter and the transducer transmits changes in BP and HR to the recorder, which converts the waves into waveforms that can be viewed on the computer screen.
After recording the hemodynamic parameters, the animals were sacrificed by sectioning the carotid artery and blood was collected in tubes. These tubes were left to stand for 30 minutes, then centrifuged. The collected supernatant was used in part to obtain hemolysis. The micro plasma tubes and hemolysates were stored at low temperature (-4°C) for subsequent biochemical assays. At the same time the animals were dissected and organs such as the aorta were removed, rinsed in physiological 0.9% NaCl solution and weighed, crushed in order to obtain organ homogenates. The width of media of aorta was measured, through a microscope (AxiosKop 40) connected to a computer. At the end of the treatment phase, the animals were left for a fasting period of 12 hours after which, they were sacrificed by cervical decapitation under light formalin anaesthesia, the blood was collected in anticoagulant tubes for the different biochemical parameters.
ii Biochemical Analysis
The protein content was determined according to the Biuret’s method using bovine serum albumin as standard [14]. The blood sugar was determined by the glucose oxidase method. Plasma triglyceride (TG), total cholesterol (TC) and high density lipoprotein (HDL-C) levels were measured using reagent kits from Fortress Diagnostics Limited (Muckamore, UK). The low density lipoprotein (LDL-C) content was calculated using the friedewald formula. Atherogenic index (AI) was calculated using the Youmbissi et al. [15].
AI = ([Total cholesterol]) / ([HDL cholesterol])
Plasma nitric oxide (NO) was evaluated by the Greiss method, based on the reduction of NO3- to NO2- in the presence of nitrate reductase [16]. An azo coloured complex is formed, produced by diazotization of sulfanilic acid (Greiss Reagent-1) with NO2- then subsequent coupling with N- (1-naphthyl) -ethylene diamine (Greiss Reagent-2).
Hemolysates were used to assess two enzymatic activities. The super-oxide dismutase (SOD) activity was evaluated according to the principle of inhibiting the oxidation of adrenaline to adrenochrom [17]. The catalase activity (CAT) was determined by measuring the decomposition of hydrogen peroxide at 570 nm [18]. The tissue glutathione (GSH) content in the tissue homogenate was measured by biochemical assay using the dithionitrobenzoic method (DTNB) [19]. The data were expressed in µg per g of aorta weight.
Lipid peroxidation level in the aorta tissue was determined by measuring the malondialdehyde (TBARS) content based on the reaction with thiobarbituric acid (TBA) [20]. Data were expressed in moles per g of liver weight using an extinction coefficient of 1.56 × 10-5 M-1 cm-1. The activity of transaminases was evaluated by measuring the plasma activities of the ALT and AST transaminases. The protocol of Reitman and Frankel was used [21]. The tests were carried out in accordance with the chronolab kit protocol. Optical densities were read at 505 nm.
VI Statistical Analyses of Data
Results were expressed as the mean ± standard deviation from the mean and percentage change. Statistical analysis was performed using Graph Pad version 8.1 for Windows. The one-way analysis of variance (ANOVA) test followed by a post hoc tukey test was used for comparison of means and percentages between groups. All values with a p <0.05 were considered significant.
Results
I Phenolic Content of the ME Leaves Aqueous Extract
The total phenolic content quantified in Manihot esculenta aqueous extract was 108.22 ± 9.43 µg catechin /gm of extract (Table 2).
Table 2: Phenolic
compound content of the aqueous extract of Manihot
esculenta leavers.
|
|
Total polyphenols (µg catechin /gm of
extract) |
|
Aqueous
extract of Manihot esculenta |
108.22
± 9.43 |
Results expressed as Mean ± SEM (n=3).
II Effect of the ME Aqueous Extract of Manihot esculenta on Some Physiological Disorders
i Effect of Manihot esculenta Leaves Aqueous Extract on Body Weight Change
Our results indicated that rats treated with distilled water had no significant change in body weight during the experimental period when compared to the initial body weight (Table 3). After 6 and 8 weeks of treatment, the body weight of rats receiving concomitantly high fat/high sucrose diet, sucrose 15% and ethanol 40° increased respectively by 10.46% and 12.65% when compared to their initial value. In the same condition, the increase was by 5.46% and 6.46% respectively as compared to the group receiving distilled water. The administration of the aqueous extract prevented the increase of body by 21.70% (p <0.05) and 17.23% (p <0.05) respectively after 6 and 8 weeks of treatment compared to rats receiving high fat/high sucrose diet and ethanol. However, there was no significant variation of the body weight recorded between the rats of group 3 when compared to rats of groups 1 and 2, respectively.
Table 3: Evolution
of body weight after administration of the aqueous extract of Manihot esculenta.
|
Week |
0 |
2 |
4 |
6 |
8 |
|
TN |
238.02 ± 7.78 |
241.76 ± 5.12 |
245.534 ± 6.86 |
248.93 ± 4.04 |
251.48 ± 9.78 |
|
TP |
237.66 ± 3.24 |
247.52 ± 5.58 |
254.63 ± 4.59# |
262.53 ± 5.74# |
267.73 ± 3.54# |
|
ME |
236.21 ± 3.94 |
241.28 ± 5.22 |
248.24 ± 4.30 |
255.56 ± 4.12* |
260.96 ± 4.63* |
TN:
group of rats receiving distilled water, TP: group of rats receiving
concomitantly high fat/high sucrose
diet and ethanol 40°, ME: group treated with M. esculenta leaf aqueous
extract in addition to high fat/high
sucrose diet and ethanol 40°. Values
are means ± SEM (n = 6). # p< 0.05 compared to TN, * p< 0.05
compared to TP.
ii Influence of Manihot esculenta Leaves Aqueous Extract on Systolic, Diastolic Blood Pressure and Heart Rate
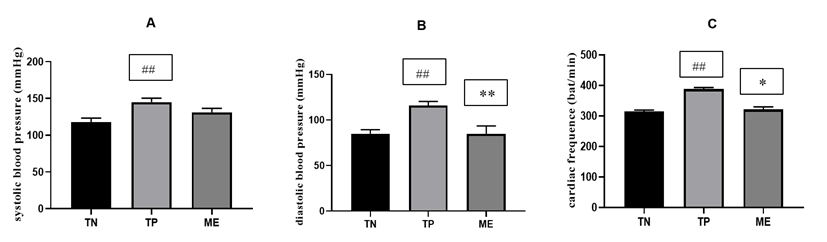
The exposure of animals to high fat/high sucrose diet combined with the administration of the alcohol and sucrose solution (inducing solution) led to a significant increased (p <0.001) of the systolic arterial pressure by 25.83%, the diastolic arterial pressure by 23.69% and the heart rate by 12.58% respectively in comparison with those of control animals. Treatment of the animal with the plant extract reduced significantly (p<0,01) the increase of diastolic pressure and heart rate (p<0,05) induced by the administration of high fat / high sucrose diet, sucrose 15% and ethanolic 40°. Whereas, no significant difference of systolic arterial pressure was found. Manihot esculenta extract have slowly reduce this parameter (Figure 1).
iii Effect of Manihot esculenta Leaves Aqueous Extract on the Width of the Media
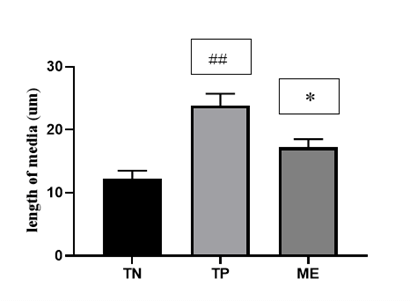
It appears from the analysis of this (Figure 1) that high fat/high sucrose diet and ethanol 40° promoted a significant increase (p <0.01) by 102.5% the width of the media in comparison to distilled water treated rats (Figure 2). Co-treatment with the leaf aqueous extract significantly (p <0.05) reduced by 32.6% the increase of the width of the media induced by high fat/high sucrose diet and ethanol 40°.
III Effects of Manihot esculenta Leaves Aqueous Extract on Some Biochemical Disorders
i Effects of Manihot esculenta Leaves Aqueous Extract on Glycaemia
From our results, no significant variation was observed between the initial and final glycaemia of rats receiving distilled water in the current study (Table 4). However, the treatment of rats with high fat/high sucrose diet and ethanol 40° produced after 8 weeks, the increase of glycaemia by 35.57% and 36.41% when compared respectively to their initial value and the final value obtained in control rats. Co-treatment of animal with the Manihot esculenta aqueous extract significantly attenuated the increase of glycaemia when compared to high fat/high sucrose diet and ethanol 40° treated animals.
Figure 1: Effect of ME treatments on systolic blood pressure, diastolic blood pressure and heart rate.
TN: group of rats receiving distilled water, TP: group of rats receiving concomitantly high fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf aqueous extract in addition to high fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). ### p <0.001 compared to TN group; and * p <0.05, ** p <0.01 compared to the TP group.
Figure 2: Effect of ME leaves aqueous extract on the width of the aortic media.
TN: group of rats receiving distilled water, TP: group of rats receiving concomitantly high fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf aqueous extract in addition to high fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). ## p <0.001, compared to TN group; and * p <0.05, compared to the TP group.
Table 4: Effect of Manihot esculenta
extract on variation of glycaemia.
|
|
initial
blood sugar mg/L |
final
blood sugar mg/L |
change
in blood sugar |
|
TN |
80.75
± 1.74 |
88.57
± 2.52 |
7.82
± 1.02 |
|
TP |
89.12
+ 3.63 |
120.82
+ 3.14## |
31.71
± 2.13 ## |
|
ME |
81.75
± 1.52 |
109.63
± 1.37* |
21.88
± 2.27* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6); ## p <0.01 compared to TN
group; and * p <0.05 compared to the TP group.
ii Effects of Manihot esculenta Leaves Aqueous Extract on Lipids Markers
Our results indicated that treatment of rats with high fat/high sucrose diet and ethanol 40° for 8 weeks significantly increased serum lipids markers (TC, TG, and LDL-c) as well as the atherogenic index of the plasma in comparison to that of distilled water treated group (Table 5). In the same time, these lipids parameters were decreased significantly (p < 0.01) in group receiving Manihot esculenta aqueous extract (about 22.67% for TC, 47.51% for LDL-c, 22.31% for TG and 45.72% for AI) as compared to group receiving high fat/high sucrose diet and ethanol 40°.
Table 5: Effect of the aqueous extract of Manihot esculenta on the plasma
levels of some markers of the lipid profile.
|
Groups |
CT (mg/dL) |
LDL-C(mg/dL) |
HDL-C (mg/dL) |
TG (mg/dL) |
Atherogenic index of plasma |
|
TN |
126.30 ± 9.51 |
59.30 ± 5.54 |
42.30 ± 4.50 |
123.27 ± 8.38 |
3.07 ± 0.25 |
|
TP |
172.76 ± 11.47## |
80.30 ± 6.84## |
32.30 ± 2.23# |
242.59 ± 6.45### |
5.38 ± 0.13# |
|
ME |
133.43 ± 8.23** |
42.30 ± 4.17** |
53.30 ± 5.21** |
188.18 ± 7.203** |
2.92 ± 0.29* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). # p <0.05; ## p <0.001;
### p <0.001 compared to TN group; and * p
<0.05 and ** p <0.01 compared to the TP group.
iii Effect of Manihot esculenta Leaves Aqueous Extract on Endogenous Antioxidants and Oxidative Stress Markers
As shown in (Table 6), high fat/high sucrose diet and ethanol 40° induced significant changes in serum markers of oxidative stress compared to control group. These changes were achieved by the decrease in SOD activity (p <0.05), catalase activity (p <0.05), GSH (p <0.05) and NO levels (p <0.01) as well as the increase of TBARS level (p <0.05). In the same condition, high fat/high sucrose diet and ethanol 40°produced the decrease of aortic NO level by 27.52% (p <0.01) and the increase of the aortic TBARS level by 65.30% compared to normal control group. Administration of Manihot esculenta aqueous extract resulted in a significant decrease (p <0.05) in serum and aortic TBARS levels as well as a significant increase (p <0.05) in serum and aortic NO levels. Similarly, serum SOD and catalase activities were significantly reduced (p <0.05) in the Manihot esculenta test group compared to high fat/high sucrose diet and ethanol 40° treated group.
Table 6:
Effect of the aqueous extract of Manihot
esculenta on oxidative stress markers.
|
|
TN |
TP |
ME |
|
Serum |
|
|
|
|
SOD
units/g of proteins |
8.91 ± 0.71 |
5.32 ± 0.86# |
9.06 ± 1.22* |
|
Catalase
mmol of H2O2/min/g
proteins |
58.43 ± 5.12 |
37.43 ± 4.69# |
49.23 ± 6.07** |
|
GSH
(umol/ml) |
41.23 ± 3.07 |
26.33 ± 2.56# |
30.74 ± 2.57 |
|
TBARS
(umol/ml) |
3.16 ± 0.52 |
6.32 ± 1.06# |
4.41 ± 1.17* |
|
NO
(mmol/ml) |
102.47 ± 9.02 |
74.43 ± 4.76## |
83.55 ± 4.47* |
|
Aorta |
|
|
|
|
SOD
units/g of proteins |
6.74 ± 0.56 |
5.19 ± 0.72# |
5.89 ± 1.07 |
|
Catalase
mmol of H2O2/min/g
proteins |
34.28 ± 3.06 |
28.94 ± 2.47# |
29.78 ± 3.19 |
|
GSH
(umol/mg tissue) |
29.83 ± 3.16 |
23.61 ± 2.11# |
26.73 ± 2.64 |
|
TBARS
(umol/mg tissue) |
2.45 ± 0.36 |
4.05 ± 0.57# |
3.04 ± 0.49* |
|
NO
(mmol/mg tissue) |
146.54 ± 5.62 |
106.21 ± 4.03## |
136.31 ± 4.23* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. # p <0.05; ##
p <0.001 compared to TN
group; and * p <0.05; ** p <0.01 compared to the TP group.
iv Effect of Manihot esculenta Leaves Aqueous Extract on Some Markers of Hepatic Cytolysis
The effects of the leaf aqueous extract of Manihot esculenta on some markers of hepatic cytolysis are shown in (Table 7). Our results show a significant increase in transaminase activities (ALT and AST) in high fat/high sucrose diet and ethanol 40° treated group compared to group receiving distilled water. Co-treatment with Manihot esculenta extract significantly reduced the activities of these markers of hepatic profile plasma AST and ALT markers compared to animals receiving high fat/high sucrose diet and ethanol 40°. Also, no significant difference was noted between the activities of AST and ALT when compared to normal control rats.
Table 7: Effects of extracts on markers
of hepatic cytolysis.
|
Groups |
ALT (U/l) |
AST (U/l) |
AST/ALT |
|
TN |
36.23
± 6.11 |
152.9
± 5.7 |
>1 |
|
TP |
42.93
± 3.6## |
163.31
± 10.8# |
>1 |
|
ME |
39.61
± 5.67* |
155
± 9.02* |
>1 |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. # p <0.05; ##
p <0.001 compared to TN
group and * p <0.05, compared to the TP group.
Discussion
Hypertension is one of the most common disorders and increases the risk of cardiovascular diseases, which are one of the main causes of death in the world. Although several modern medicines are used to manage hypertension, numerous studies have demonstrated the effectiveness of medicinal plant extracts or their fractions in the treatment of this diseases [22]. Manihot esculenta leaf used in the present study is commonly eaten by people in Africa. In Cameroon, fresh cassava leaf juice in combination with milk is often recommended in the management of anemia [23]. In addition, the consumption of cassava leaves is often recommended to patients suffering from pathologies such as malaria, typhoid fever to name a few. Also, it should be mentioned that several epidemiological studies have established a significant correlation between the diets of rural areas and the relatively low rate of patients with high blood pressure [24]. Therefore, the present study is aimed to evaluate the protective effects of the leaf aqueous extract of Manihot esculenta Crantz on high-calorie diet combined with alcohol and sucrose ingestion induced hypertension in rats.
The health benefits of cassava leaves have been suggested by reports on their nutritional, mineral, and phytochemicals contents. Indeed, the presence of secondary metabolites such as terpenoids, flavonoids and polyphenolic classes of compounds in Manihot esculenta have been demonstrated previously [25, 26]. In agreement, our results demonstrate the presence of polyphenols and flavonoids in the leaf aqueous extract of Manihot esculenta [27, 28]. The presence of these compounds may partly explain the protective effects of Manihot esculenta leaf against the development of various chronic diseases such as cardiovascular diseases [29]. Results of this study also show that the administration of the plant extract significantly prevented the increase of body weight after 6 and 8 weeks when compared to rats receiving only high fat/high sucrose diet and ethanol. This reduction of body weight may be attributed to the phytochemical compounds which avoid weight gain and fat accumulation via increasing fat metabolism and inhibiting fat absorption [30]. Current finding showed that the exposure of rats to high fat/high sucrose diet and ethanol 40° during 8 weeks significantly increased both systolic and diastolic arterial blood pressures as well as the heart rate when compared to control animals receiving distilled water. The increase of these hemodynamic markers may be associated to the onset and/or worsening of some cardiovascular risk factors such as such as insulin resistance, oxidative stress, atherogenicity and dyslipidemia [31, 32].
Treatment with the leaf aqueous extract of Manihot esculenta significantly decrease the heart rate and diastolic blood pressure, but did not significantly reduce systolic blood pressure. These results are in line with previous study that suggesting that the leaves of Manihot esculenta are rich in phytochemicals, which are valuable as natural antioxidants, and their consumption has been linked to reducing the risks of hypertension [33]. Present findings also showed that the width was significantly greater in hypertensive animals than in normal control group. This result is similar to previous studies that demonstrated that increased width was associated with greater aortic stiffness which is closely linked to increased risk of hypertension [34]. The co-treatment of animals with the leaf aqueous extract significantly prevented the increase of the median width induced by high fat/high sucrose diet and ethanol 40°. This result suggests that the leaf aqueous extract of Manihot esculenta may contain some biochemical components that are able to reduce the blood pressure by improving vascular remodeling as previously reported [35].
In this study, we observed that the blood glucose level of rats treated with high fat/high sucrose diet and ethanol 40°was significantly greater in comparison with the normal control group. This increase can directly impair vasodilation and increase oxidative. The sum of these effects is the impaired autoregulation of vascular tone, increased vascular resistance and blood pressure elevation [36]. Co-treatment of rats with the leaf aqueous extract of Manihot esculenta significantly prevented the increase of blood glucose induced by high fat/high sucrose diet and ethanol 40°. This result was consistent with previous works reporting that the consumption of cassava leaves rich in phytochemicals, such as phenolic acids, saponins, stilbenes, carotenoids and flavonoids, may act to prevent non-communicable diseases such as cardiovascular disease, stroke and diabetes [37]. Along with hyperglycemia, dyslipidemia also affect vascular tone through imbalance of vascular tone regulators.
Our results also revealed that high fat/high sucrose diet and ethanol 40° feeding significantly increase the serum total cholesterol, triglycerides, LDL-cholesterol as well as the atherogenic index while the HDL concentration decrease in comparison to distilled water treated group. These variations in the serum lipid profile are in line with previous studies which have shown that excessive consumption of fat, sugar and/or alcohol largely contributes to the development of dyslipidemia [38, 39]. Indeed, it has been reported that dyslipidemia causes endothelial damage and consequently, reduced vasodilator capacity and increased blood pressure [40]. Compared to hypertensive animals, the administration of the leaf extract significantly induced on the one hand a decrease of the total cholesterol, LDL cholesterol and triglyceride levels, and in the other hand an increase of the HDL level. The observed ameliorative effects on lipid profile suggests that the leaf aqueous extract of Manihot esculenta may contain phytochemicals such as polyphenols that are able to prevent dyslipidemia [41].
Previous studies demonstrate that oxidative stress plays an important role in the pathogenesis and development of various diseases such as hypertension, dyslipidemia, diabetes mellitus, atherosclerosis and other chronic diseases [42]. In the present work, high fat/high sucrose diet and ethanol 40° significantly decrease the serum SOD and catalase activities as well as GSH and NO levels when compared to normal control rats. In the same condition, these hypertensive agents significantly decrease the aortic NO level and increase the aortic TBARS level. These results are consistent with previous studies that have shown that the antioxidant defense system decline in high fat, high sucrose and/or ethanol fed animals [43-45]. In contrast, treatment with the plant extract significantly ameliorate the antioxidative parameters evaluated in the current study as compared to animals receiving concomitantly high fat/high sucrose diet and ethanol 40°. These results suggest that the leaf aqueous extract of Manihot esculenta may exert its antihypertensive potential partly via its its ability to prevent oxidative stress and to restore the antioxidant properties.
In this study, we also investigated the effect of M. esulenta leaf aqueous extract on some markers of hepatic functions. Interestingly, our results showed a significant increase in transaminase activities (ALT and AST) following high fat/high sucrose diet and ethanol 40° feeding compared to group receiving distilled water. As mentioned in several studies, an increase in the activity of the above enzymes is an indication of severe liver damage [46, 47]. Current findings indicate that the hepatoprotective effect of leaf aqueous extract of Manihot esculenta could be associated to its ability to decrease the above-mentioned enzymatic markers of liver functions.
Conclusion
The treatment with the leaf aqueous extract of Manihot esculenta produced potent beneficial effects against high fat/high sucrose diet and ethanol 40°induced hypertension in rats. These effects may be attributed to the presence of some phytochemicals such as polyphenols that are able to improve vascular function and to exhibit hypoglycemic, hypolipidemic, hepatoprotective and antioxidant effects. These results justify the traditional use of Manihot esculenta leaves to reduce the risk of developing hypertension. However, further studies are needed to explore other metabolic pathways that may explain the observed in vivo effect on blood pressure.
Author Contributions
Bibi Farouck and Eric Beyegue conceived and designed the review, Bibi Farouck and Eric Beyegue wrote the review. Clemence Mvogo and Eric Beyegue revised the review. Danielle Claude Bilanda as supervisor. All authors discussed and approved the final version.
Conflicts of Interest
None.
Abbreviations
TN: Negative control group feed with standard diet
TP: Positive control group feed with high fat/ high sucrose diet
ME: M. esculenta aqueous extracts
Article Info
Article Type
Research ArticlePublication history
Received: Sat 24, Jun 2023Accepted: Thu 20, Jul 2023
Published: Mon 07, Aug 2023
Copyright
© 2023 Bibi-Farouck ABOUBAKAR OUMAROU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2023.02.01
Author Info
Bibi-Farouck ABOUBAKAR OUMAROU Eric BEYEGUE Clemence MVONGO Martin Thierry BELLA NDZANA Danielle Claude BILANDA
Corresponding Author
Bibi-Farouck ABOUBAKAR OUMAROUDepartment of Physiological Sciences and Biochemistry, Faculty of Medicine and Biomedical Sciences, University of Garoua, Garoua-Cameroon
Figures & Tables
Table 1: Composition of the
experimental diets (g/kg diet) [48].
|
Ingredients |
Standard died (SD) |
High fat/High sucrose died
(HFHS) |
|
Milk
|
/ |
145 g |
|
Sucrose |
/ |
290 g |
|
Corn
starch of blew |
216 g |
188 g |
|
Corn
starch of maze |
324 g |
237 g |
|
Margarine
|
/ |
143,3 g |
|
Palm
oil |
50 g |
55 g |
|
Cellulose
powder |
/ |
30 g |
|
Mineral
mixture |
20 g |
20 g |
|
Vitamin
mixture |
5 g |
5 g |
Table 2: Phenolic
compound content of the aqueous extract of Manihot
esculenta leavers.
|
|
Total polyphenols (µg catechin /gm of
extract) |
|
Aqueous
extract of Manihot esculenta |
108.22
± 9.43 |
Results expressed as Mean ± SEM (n=3).
Table 3: Evolution
of body weight after administration of the aqueous extract of Manihot esculenta.
|
Week |
0 |
2 |
4 |
6 |
8 |
|
TN |
238.02 ± 7.78 |
241.76 ± 5.12 |
245.534 ± 6.86 |
248.93 ± 4.04 |
251.48 ± 9.78 |
|
TP |
237.66 ± 3.24 |
247.52 ± 5.58 |
254.63 ± 4.59# |
262.53 ± 5.74# |
267.73 ± 3.54# |
|
ME |
236.21 ± 3.94 |
241.28 ± 5.22 |
248.24 ± 4.30 |
255.56 ± 4.12* |
260.96 ± 4.63* |
TN:
group of rats receiving distilled water, TP: group of rats receiving
concomitantly high fat/high sucrose
diet and ethanol 40°, ME: group treated with M. esculenta leaf aqueous
extract in addition to high fat/high
sucrose diet and ethanol 40°. Values
are means ± SEM (n = 6). # p< 0.05 compared to TN, * p< 0.05
compared to TP.
Table 4: Effect of Manihot esculenta
extract on variation of glycaemia.
|
|
initial
blood sugar mg/L |
final
blood sugar mg/L |
change
in blood sugar |
|
TN |
80.75
± 1.74 |
88.57
± 2.52 |
7.82
± 1.02 |
|
TP |
89.12
+ 3.63 |
120.82
+ 3.14## |
31.71
± 2.13 ## |
|
ME |
81.75
± 1.52 |
109.63
± 1.37* |
21.88
± 2.27* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6); ## p <0.01 compared to TN
group; and * p <0.05 compared to the TP group.
Table 5: Effect of the aqueous extract of Manihot esculenta on the plasma
levels of some markers of the lipid profile.
|
Groups |
CT (mg/dL) |
LDL-C(mg/dL) |
HDL-C (mg/dL) |
TG (mg/dL) |
Atherogenic index of plasma |
|
TN |
126.30 ± 9.51 |
59.30 ± 5.54 |
42.30 ± 4.50 |
123.27 ± 8.38 |
3.07 ± 0.25 |
|
TP |
172.76 ± 11.47## |
80.30 ± 6.84## |
32.30 ± 2.23# |
242.59 ± 6.45### |
5.38 ± 0.13# |
|
ME |
133.43 ± 8.23** |
42.30 ± 4.17** |
53.30 ± 5.21** |
188.18 ± 7.203** |
2.92 ± 0.29* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). # p <0.05; ## p <0.001;
### p <0.001 compared to TN group; and * p
<0.05 and ** p <0.01 compared to the TP group.
Table 6:
Effect of the aqueous extract of Manihot
esculenta on oxidative stress markers.
|
|
TN |
TP |
ME |
|
Serum |
|
|
|
|
SOD
units/g of proteins |
8.91 ± 0.71 |
5.32 ± 0.86# |
9.06 ± 1.22* |
|
Catalase
mmol of H2O2/min/g
proteins |
58.43 ± 5.12 |
37.43 ± 4.69# |
49.23 ± 6.07** |
|
GSH
(umol/ml) |
41.23 ± 3.07 |
26.33 ± 2.56# |
30.74 ± 2.57 |
|
TBARS
(umol/ml) |
3.16 ± 0.52 |
6.32 ± 1.06# |
4.41 ± 1.17* |
|
NO
(mmol/ml) |
102.47 ± 9.02 |
74.43 ± 4.76## |
83.55 ± 4.47* |
|
Aorta |
|
|
|
|
SOD
units/g of proteins |
6.74 ± 0.56 |
5.19 ± 0.72# |
5.89 ± 1.07 |
|
Catalase
mmol of H2O2/min/g
proteins |
34.28 ± 3.06 |
28.94 ± 2.47# |
29.78 ± 3.19 |
|
GSH
(umol/mg tissue) |
29.83 ± 3.16 |
23.61 ± 2.11# |
26.73 ± 2.64 |
|
TBARS
(umol/mg tissue) |
2.45 ± 0.36 |
4.05 ± 0.57# |
3.04 ± 0.49* |
|
NO
(mmol/mg tissue) |
146.54 ± 5.62 |
106.21 ± 4.03## |
136.31 ± 4.23* |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. # p <0.05; ##
p <0.001 compared to TN
group; and * p <0.05; ** p <0.01 compared to the TP group.
Table 7: Effects of extracts on markers
of hepatic cytolysis.
|
Groups |
ALT (U/l) |
AST (U/l) |
AST/ALT |
|
TN |
36.23
± 6.11 |
152.9
± 5.7 |
>1 |
|
TP |
42.93
± 3.6## |
163.31
± 10.8# |
>1 |
|
ME |
39.61
± 5.67* |
155
± 9.02* |
>1 |
TN: group of rats receiving distilled water, TP:
group of rats receiving concomitantly high
fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf
aqueous extract in addition to high
fat/high sucrose diet and ethanol 40°. # p <0.05; ##
p <0.001 compared to TN
group and * p <0.05, compared to the TP group.
TN: group of rats receiving distilled water, TP: group of rats receiving concomitantly high fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf aqueous extract in addition to high fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). ### p <0.001 compared to TN group; and * p <0.05, ** p <0.01 compared to the TP group.
TN: group of rats receiving distilled water, TP: group of rats receiving concomitantly high fat/high sucrose diet and ethanol 40°, ME: group treated with Manihot esculenta leaf aqueous extract in addition to high fat/high sucrose diet and ethanol 40°. Values are means ± SEM (n = 6). ## p <0.001, compared to TN group; and * p <0.05, compared to the TP group.
References
1.
Sudharsanan N,
Geldsetzer P (2019) Impact of coming demographic changes on the number of
adults in need of care for hypertension in Brazil, China, India, Indonesia,
Mexico, and South Africa: a modeling study. Hypertension
73: 770-776. [Crossref]
2.
El Hilaly J,
Amarouch MY, Morel N, Lyoussi B, Quetin Leclercq J (2021) Ajuga iva water
extract antihypertensive effect on stroke-prone spontaneously hypertensive
rats, vasorelaxant effects ex vivo and in vitro activity of fractions. J Ethnopharmacol 270: 113791. [Crossref]
3.
Belhayara MI,
Mellouk Z., Hamdaoui MS, Bachaoui M, Kheroua O et al. (2020) The metabolic syndrome: emerging novel insights
regarding the relationship between the homeostasis model assessment of insulin
resistance and other key predictive markers in young adults of Western Algeria.
Nutrients 12: 727. [Crossref]
4.
Weber MA, Schiffrin
EL, White WB, Mann S, Lindholm LH et al. (2014) Clinical practice guidelines
for the management of hypertension in the community: a statement by the
American Society of Hypertension and the International Society of Hypertension.
J Clin Hypertens 16: 14-26. [Crossref]
5.
Konta EM, Almeida
MR, do Amaral CL, Darin JDC, dee Rosso VV et al (2014) Evaluation of the
antihypertensive properties of yellow passion fruit pulp (Passiflora edulis
sims f. flavicarpa deg.) in spontaneously hypertensive rats. Phyther. Res 28: 28-32. [Crossref]
6. Kumar KV, Das U (1993) Are free radicals involved in
the pathobiology of human essential hypertension? Free Radical Research
19: 59-66 [Crossref]
7.
Shamsi A, Ahmed A,
Khan MS, Husain FM, Bano B (2020) Rosmarinic acid restrains protein glycation
and aggregation in human serum albumin: multi spectroscopic and microscopic
insight-possible therapeutics targeting diseases. Int J Biol Macromol 161:
187-193. [Crossref]
8.
Bayan L, Koulivand
PH, Gorji A (2014) Garlic: a review of potential therapeutic effects. Avicenna J Phytomed 4: 1-14. [Crossref]
9.
Amiot MJ, Riva C, Vinet A (2016) Effects of dietary
polyphenols on metabolic syndrome features in humans: a systematic review. Obes
Rev 17: 573-586. [Crossref]
10. Incalza MA, D'Oria R, Natalicchio A, Perrini S,
Laviola L et al (2018) Oxidative stress and reactive oxygen species in
endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 100: 1-19. [Crossref]
11. Okoro IO (2020) Two Extracts from Manihot esculenta Leaves Efficiently Inhibit α-Glucosidase and
α-Amylase: A New Approach for the Management of Diabetes. IJT, 14:
131-138.
12. Singleton VL, Rossi JA (1965) Colorimetry of total
phenolics with phosphomolybdicphosphotungstic acid reagents. Amer. J. Enol.
Viticult 16: 144-158.
13. Bilanda DC,
Bidingha RAG, Djomeni Dzeufiet PD, Fouda YB, Ngapout RF et al. (2020)
Antihypertensive and antidiabetic activities of Erythrina senegalensis DC
(Fabaceae) stem bark aqueous extract on diabetic hypertensive rats. J
Ethnopharmacol 246: 112200. [Crossref]
14. Gornall AC, Bardawill CJ, David MM (1949) Determination
of Serum Proteins by means of the Biuret reaction. J. Biol. Chem 177: 751-766. [Crossref]
15. Youmbissi TJ, Djoumessi S, Nouedoui C, Ndobo P, Meli J
(2001) Profil lipidique d'un groupe d'hypertendus camerounais noirs africains. Médecine d'Afrique
Noire 48:
305-314.
16. Sreejayan, Rao MN (1997) Nitric oxide scavenging by
curcuminoids. J Pharm. Pharmacol 49:
105-107. [Crossref]
17. Misra HP, Fridowich I (1972) The generation of
superoxide radical during the autoxidation of haemoglobin. J Biol Chem 247: 6960-6962. [Crossref]
18. Sinha KA (1972) Colorimetric assay of catalase. Anal
Biochem 47: 389-394. [Crossref]
19. Ellman G (1959) Quantitative Determination of Peptides
by Sulfhydryl (-SH) Groups. Arch Biochem Biophys 82: 70-77.
20. Yagi K (1976) Simple Fluorometric Assay for
lipoperoxyde in blood plasma. Biochem Med 15: 212-216. [Crossref]
21. Reitman S, Frankel S (1957)
Dosage des transaminases sériques. American Journal of Clinical Pathology
28: 56.
22. Belemnaba L,
Nitiéma M, Ilboudo S, Ouédraogo GG, Ouédraogo N et al. (2021) Preclinical
Evaluation of the Antihypertensive Effect of an Aqueous Extract of Anogeissus
leiocarpa (DC) Guill et Perr. Bark of Trunk in L-NAME-Induced Hypertensive Rat.
J Exp Pharmacol 13: 739-754. [Crossref]
23. Temesgen Zekarias T, Bakalo B, Tamirat H (2019).
Medicinal, Nutritional and Anti-Nutritional Properties of Cassava (Manihot esculenta): A Review. Academic Journal of Nutrition 8: 34-46.
24. Ntentie FR, Ngondi JL, Azantsa KBG, Santy EV, Dimodi
HT et al. (2014) Urbanization and Metabolic Syndrome in Cameroon: Alertness on
Less Urbanised Areas. Endocrinol Metab Synd 3: 137.
25. Boukhers I, Boudard F, Morel S, Servent A, Portet K et
al. (2022) Nutrition, Healthcare Benefits and Phytochemical Properties of
Cassava (Manihot esculenta) Leaves Sourced from Three Countries (Reunion, Guinea, and Costa Rica).
Foods 11: 20-27. [Crossref]
26. Jampa M, Sutthanut K, Weerapreeyakul N, Tukummee W,
Wattanathorn J et al (2022) Multiple Bioactivities of Manihot esculenta Leaves: UV Filter, Anti-Oxidation, Anti-Melanogenesis,
Collagen Synthesis Enhancement, and Anti-Adipogenesis. Molecules 27:
1556. [Crossref]
27. Clark JL, Zahradka P, Taylor CG (2015) Efficacy of
flavonoids in the management of high blood pressure. Nutr. Rev 73: 799-822. [Crossref]
28. Bahekar SE, Kale RS (2016). Evaluation of antioxidant
activity of Manihot esculenta Crantz in wistar rats. J Pharm Bioallied Sci 8: 119-123. [Crossref]
29. Mutha RE, Tatiya AU, Surana SJ (2021) Flavonoids as natural phenolic compounds and their
role in therapeutics: an overview. Futur J Pharm Sci 7: 25. [Crossref]
30. Elpasty SSA, Helal EGE, Mansoury MMS, Algendy AMM
(2022) Impact of Green Coffee Extract on Body Weight and Physiological
Indicators of Metabolic State in Obese Male Rats. Egypt J Chem 65:
715-723.
31. Bilanda DC, Djomeni Dzeufiet PD, Bopda OM, Kamtchouing
P, Dimo T (2018) Allablanckia floribunda hypotensive activity on ethanol
induced hypertension in rats The Journal of Phytopharmacology 7:
146-151.
32. Soyung L, Jang S, Kim JY, Kim I (2022) Dahl
Salt-Resistant Rat Is Protected against Hypertension during Diet-Induced
Obesity. Nutrients 14: 38-43. [Crossref]
33. Oghenevwodokohwo OI (2020) Two Extracts from Manihot
Esculenta Leaves Efficiently Inhibit α-Glucosidase and α-Amylase: A New
Approach for the Management of Diabetes. Iranian Journal of Toxicology
14: 131-138.
34. Taylor LE, Gillis EE, Musall
JB, Babak B, Sullivan JC (2018) High-fat diet-induced hypertension is associated
with a pro-inflammatory Tcell profile in male and female Dahl salt-sensitive
rats. Am J Physiol Heart Circ Physiol 315: H1713-H1723. [Crossref]
35. Park SY, Jeong EW, Yang YS, Kim HJ, Go GW et al (2021)
Finger Millet Ethanol Extracts Prevent Hypertension by Inhibiting the
Angiotensin-Converting Enzyme Level and Enhancing the Antioxidant Capacity in
Spontaneously Hypertensive Rats. Antioxidants 10: 1766. [Crossref]
36. Kim DY, Piao J, Hong HS (2021) Substance-P Inhibits
Cardiac Microvascular Endothelial Dysfunction Caused by High Glucose-Induced
Oxidative Stress. Antioxidants 10: 1084. [Crossref]
37. Laya A, Koubala BB, Negi PS (2022) Antidiabetic
(α-amylase and α-glucosidase) and anti-obesity (lipase) inhibitory activities
of edible cassava (Manihot esculenta Crantz) as measured
by in vitro gastrointestinal digestion: effects of phenolics and harvested
time. International journal of food
properties 25: 492-508.
38. Angassa
D, Solomon S, Seid A. (2022) Factors associated with dyslipidemia and its
prevalence among Awash wine factory employees, Addis Ababa, Ethiopia: a
cross-sectional study. BMC
Cardiovasc Disord 22: 22. [Crossref]
39. Iqbal
A, Najam R, Simjee S, Ishaqui AA, Ahmed SA, et al. Cepharanthine Action in
Preventing Obesity and Hyperlipidemia in Rats on a High-Fat High Sucrose Diet. Saudi Pharm J 30:1683-1690. [Crossref]
40. Lin Y-H, Liu Y-H, Wu D-W, Su H-M, Chen S-C (2022).
Dyslipidemia Increases the Risk of Incident Hypertension in a Large Taiwanese
Population Follow-Up Study. Nutrients 14: 3277. [Crossref]
41. Ramchoun M, Khouya T, Harnafi H, Alem C, Benlyas M et
al. (2020) Effect of polyphenol, flavonoid, and saponin fractions from Thymus
atlanticus on acute and chronic hyperlipidemia in mice. Future Journal of
Pharmaceutical Sciences 6: 69.
42. Khutami C, Sumiwi SA, Khairul Ikram NK, Muchtaridi M
(2022) The Effects of Antioxidants from Natural Products on Obesity,
Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. Int J Mol
Sci 23: 2056 [Crossref]
43. Hamid A, Ibrahim FW, Ming TH, Nasrom MN, Eusoff N et
al. Zingiber zerumbet L. (Smith) extract alleviates the ethanol-induced brain
damage via its antioxidant activity. BMC Complement Altern Med 18: 101.
[Crossref]
44. Lasker S, Rahman MM, Parvez F, Zamila M, Miah P et al.
(2019) High-fat diet-induced metabolic syndrome and oxidative stress in obese
rats are ameliorated by yogurt supplementation. Sci Rep 9: 20026. [Crossref]
45. Alptekin Ö, Tükel SS, Turan B, Kuyucu Y (2022)
Alterations in Antioxidant Defense Systems and Metal Profiles in the Liver of
Rats with Metabolic Syndrome Induced with High-Sucrose Diet. JOTCSA 9:
13-20.
46. Jin X, Li Y, Li J, Cheng L, Yao Y et al. (2022) Acute
bone damage through liver-bone axis induced by thioacetamide
in rats. BMC Pharmacol Toxicol 23: 29. [Crossref]
47. Azimi M, Mehrzad J, Ahmadi E, Orafei M, Aghaie F et
al. (2022) The Effect of Thymus vulgaris on Hepatic Enzymes Activity and
Apoptosis-Related Gene Expression in Streptozotocin-Induced Diabetic Rats. Evid
Based Complement Alternat Med 2022: 2948966. [Crossref]
48. Ngondi JL, Mbong Angie MA, Ndongang ES, Nguimkeng
Signing B, Oben JE (2014) Modulatory effect of a polyphenolic rich extract of
Dacryodes macrophylla berries on biomarkers of metabolic syndrome and oxidative
stress in rats fed High Fat- High Sucrose diet. American Journal of Pharmacy and Health Research 2: 2321-3647.
49. Mata Greenwood E, Chen DB (2008) Racial differences in
nitric oxide-dependent Vasorelaxation. Reprod Sci 15: 9-25. [Crossref]
50. Bernatova I (2014) Endothelial dysfunction in
experimental models of arterial hypertension: cause or consequence? Biomed
Res Int 2014: 598271. [Crossref]
51. Ibrahim B, Nkoulémbéné CA, Mounguengui S, Lépengué AN,
Azizet YI (2015) Antihypertensive potential of aqueous extract of Nephrolepis
biserrata leaves on toad aorta. Med Aromat Plants 5: 220-228.
52. Wobeto C, Corrêa AD, Abreu CMPD, Santos CDD, Abreu JRD
(2006) Nutrients in the cassava (Manihot
esculenta Crantz) leaf meal
at three ages of the plant. Food Sci. Technol 26: 865-869.
53. Baradaran A, Nasri H, Rafieian Kopaei M (2014)
Oxidative stress and hypertension: Possibility of hypertension therapy with
antioxidants. J Res Med Sci 19: 358-367. [Crossref]
54. Ngene JP, Ngoule CC, Kidik CMP, Ottou PM, Dibong SD et
al. (2015) Importance dans la pharmacopée traditionnelle des plantes à
flavonoïdes vendues dans les marchés de Douala est (Cameroun). Journal of
Applied Biosciences 88: 8194-8210.
55. Fasuyi AO (2005) Nutrient composition and processing
effects on cassava leaf (Manihot esculenta, Crantz) antinutrients. Pakistan
Journal of Nutrition 4: 37-42.
56. Okeke CU, Iweala E (2007) Antioxidant profile of
Dioscorea rotundata, Manihot esculenta, Ipoemea batatas, Vernonia
amygdalina and Aloe vera. J Med Res Technol 4: 4-10.
57. Miladiyah I (2011) Analgesic activity of ethanolic
extract of Manihot esculenta Crantz leaves in mice. Universa medicina 30:
3-10.
58. Friedewald WT, Levy RI, Fredrickson DS (1972)
Estimation of the concentration of low-density lipoprotein cholesterol in
plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499-502.
[Crossref]
59. World Health Organization (2000) General guidelines for methodologies on research and evaluation of traditional medicine (No. WHO/EDM/TRM/2000.1). World Health Organization.
60. Lackland DT, Voeks JH (2014) Metabolic syndrome and hypertension: regular exercise as part of lifestyle management. Curr Hypertens Rep 16: 492. [Crossref]
