Prolonged Anhepatic Phase after Liver Transplantation Failure Followed by ABO Incompatible Liver Transplantation: A Case Report and Review of the Literature
A B S T R A C T
Objective: To describe the experience with a multimodal therapeutic approach in a patient who developed toxic liver syndrome and fulminant hepatic failure following orthotopic liver transplantation (OLT) as a result of occlusion of the portal vein.
Setting: Department of Intensive Care.
Patient: A patient with liver cirrhosis secondary to autoimmune hepatitis and primary biliary cirrhosis who underwent orthotopic liver transplantation (OLT).
Interventions: Transplant hepatectomy, plasmapheresis and retransplantation.
Case Report: A 39-year-old man underwent an elective OLT. A routine postoperative doppler ultrasound examination a few hours after surgery revealed portal vein thrombosis. Attempts at recanalization failed, and the patient developed acute fulminant liver failure, which remained resistant to supportive therapy. A transplant hepatectomy was performed 9 hours later and plasmapheresis started. Following a 10-hour anhepatic period, the patient received a second liver, from an ABO-incompatible donor. The patient underwent column plasmapheresis and subsequent splenectomy to remove anti-B antibody to preserve the incompatible transplanted liver from immunogenic complications. The patient spent a total of 21 days in the Intensive Care Unit (ICU) before being discharged to a step-down ward.
Conclusion: Our experience suggests that multimodal therapy, including transplant hepatectomy, plasmapheresis and retransplantation of an even non-ABO compatible liver may result in the successful outcome in patients with acute fulminant liver failure complicating OLT.
Keywords
Orthotopic liver transplantation, transplant hepatectomy, prolonged anhepatic phase, plasmapheresis and retransplantation
Introduction
The anhepatic phase is defined as the time from the physical removal of the liver from the recipient to the recirculation of the graft [1]. The management of the anhepatic phase is a matter of debate. It is still a great challenge for intensive care physicians to manage this condition properly. Orthotopic liver transplantation (OLT) is presently the most accepted treatment for end-stage liver disease with 1-year survival in recipients around 90 % [2]. Vascular complications following OLT are rare, being around 7% in various series of deceased OLT [3]. However, they are one of the most dreaded complications with a high incidence of both graft loss and mortality, as they compromise the blood flow of the transplant (either inflow or outflow). Khalaf in 2010 reported that patients who presented with vascular complications had significantly inferior graft and patient survival rates [4]. Portal vein complications (PVCs) following liver transplantation are even less common, occurring in 1% to 3% of patients. These complications, which are more common with split liver and pediatric transplantation, are associated with particularly high morbidity, graft loss and mortality, and their successful management constitute a major therapeutic challenge.
We describe here our experience with an anhepatic patient in ICU after the failure of liver transplantation.
Case Presentation
A 39-year-old man suffering from liver cirrhosis secondary to overlapping autoimmune-induced hepatitis and primary biliary cirrhosis was admitted to the ICU department after undergoing elective OLT from a heart-beating, brain-dead donor. A split liver procedure was performed, with the right extended liver lobe graft transplanted to the patient. Good liver perfusion of the liver after transplantation was noted intraoperatively, and hemodynamic stability was observed during the entire procedure, with no requirement for blood products. Anti-interleukin-2 receptor monoclonal antibody (Basiliximab), was administered at the end of the surgery.
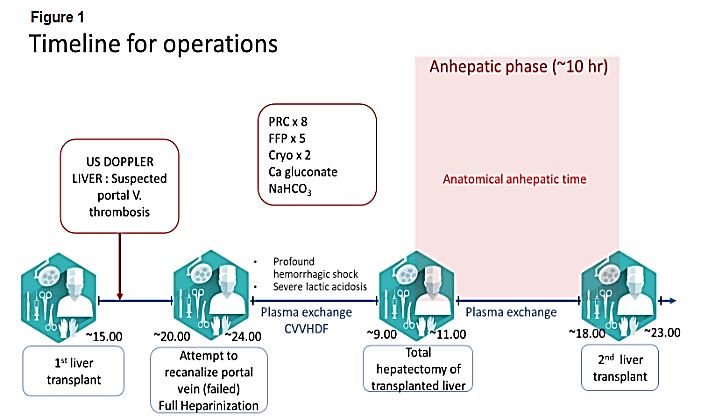
The patient was extubated immediately following surgery and transferred to the ICU. A routine bedside-doppler ultrasound of the abdomen performed 2 hours-postoperatively revealed thrombosis of portal vein and absence of flow in the portal vein. The patient was immediately transferred to the operating room where the surgical team revealed a thrombus that fills the wall of anastomose, thrombectomy and recanalization of the portal vein was attempted but failed (Figure 1). The liver graft was noted to be edematous and showed early signs of necrosis, and the patient was listed for emergency retransplantation.
Figure 1: US DOPPLER LIVER: ultrasound doppler liver; PRC: packed red blood cells; FFP: fresh frozen plasma; Cryo: cryoprecipitate; Ca gluconate: calcium gluconate; NaHCO3: sodium bicarbonate; ~ means time in hours
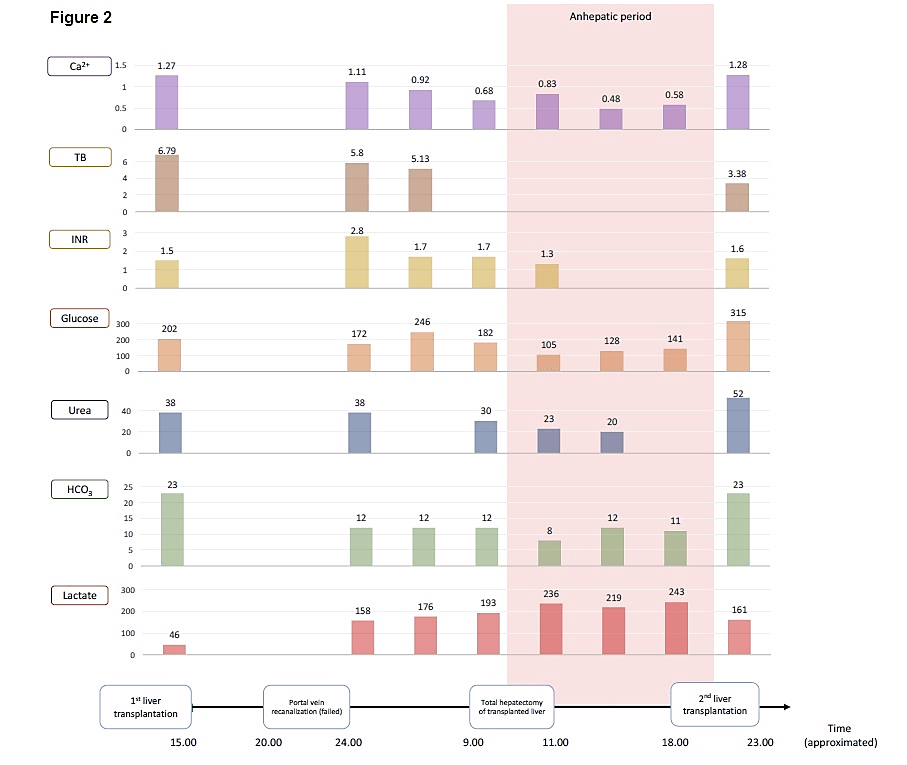
On return to the ICU, full heparinization to prevent further thrombosis of the graft vessels was started. Infusions of norepinephrine and vasopressin were required to maintain hemodynamic stability. Shortly thereafter, massive bleeding was observed from all the intra-abdominal drains and the hemoglobin decreased from 9.3 to 6 g/dl. Heparin was discontinued, and protamine sulphate given in addition to blood component transfusions. Blood examination revealed a severe metabolic acidosis with a markedly elevated lactate level (Figure 2). As the patient remained anuric and acidotic, continuous veno-venous hemo-dia-filtration (CVVHDF) was commenced. In addition, considering the fulminant nature of the liver failure, high volume plasmapheresis (HVP) using fresh frozen plasma was commenced. Liver enzymes and coagulation tests were extremely abnormal (Figure 2).
Continuous infusions of dextrose were required to maintain normoglycemia, and prophylactic broad-spectrum antibiotic and antifungal therapy were started. In view of the ongoing unresponsive severe hemodynamic instability and uncontrolled bleeding, a total hepatectomy with a temporary portocaval shunt was performed 9 hours after the second procedure and 24 hours after the first ICU admission.
Following surgery, immunosuppressive therapy was withheld. Following a period of 23 hours without a functional liver, including 10 hours of formal anhepatic state, the patient underwent a second liver transplantation from an ABO-Incompatible donor. A perioperative ultrasound doppler of the transplanted liver revealed normal blood flow in the hepatic vein and artery. A few hours later, the patient was partly weaned from hemodynamic support. No further blood product transfusions were required. Immunosuppressive therapy was resumed with tacrolimus. Following the acute care through the second surgery, more complications due to ABO incompatibility were faced and treated.
Figure 2: Represent the levels of Ca²+(ionised calcium ;mmol/l ), TB( total bilirubin; mg/dl), INR ( International normalized ratio), glucose(%mg), urea (mmol/l), HCO3-( bicarbonate;mmol/l ) and lactate(mmol/l) between the first split transplantation to the all period of anhepatic phase.
Discussion
We have presented a case, who developed a toxic liver syndrome (TLS) following OLT secondary occlusion of the portal vein. TLS is characterized by complete liver necrosis, which in our patient was associated with cardiovascular shock, renal and respiratory failure requiring respiratory support, hemodialysis and mechanical ventilation [5]. The pathophysiology remains unclear but may involve toxic metabolites released from the necrotic ischemic liver, including cytokines or cardio-suppressive factors that play a role after ischemia reperfusion injury syndrome [5, 6]. It is invariably fatal because of liver failure, and there are no documented cases of reversible TLS. In order to sustain the fulminant hepatic failure while awaiting a repeat liver graft, plasmapheresis was used as a support liver device.
In the setting of acute liver failure (ALF), therapeutic plasma exchange (TPE) with fresh frozen plasma (FFP) as the replacement fluid has been shown to improve laboratory measures of coagulation without the risk of excessive fluid loading. Furthermore, liver enzyme and ammonia levels may be reduced [7]. A recent study showed that high volume plasmapheresis treatment improves outcomes in patients with ALF by increasing liver transplant-free survival which is thought to be attributable to attenuation of innate immune activation and amelioration of multi-organ dysfunction [8]. Standard-volume plasma exchange has also been utilized for in the treatment of acute and acute-on-chronic liver failure and has demonstrated a greater improvement in the post-plasma exchange CLIF-C acute-on-chronic -liver failure (ACLF) score when compared to patients who did not undergo plasma exchange [9].
The only effective treatment for TLS, however, is the removal of the nonfunctioning and necrotic liver, which removes the source of the vasoactive agents responsible for TLS. Our patient underwent a total hepatectomy with a portacaval shunt that permitted the stabilization of hemodynamic and metabolic functions and decompression of the portal venous system. The safe duration of the an-hepatic phase is not well known. Durations ranging from 41 hours, 60 hours and a range of 6.58 to 72.5 hours were reported by Ringe, Bentdal, and Oldhafer, respectively [10-12]. Oldhafer KJ, et al. described the relation between outcome and duration of anhepatic period in patients undergoing a two-stage hepatectomy and subsequent liver transplantation [12]. Of 20 such patients, 6 survived with a follow up of 1 to 8 years. The survivors had a shorter duration of anhepatic phase. More than 65 minutes of anhepatic phase is typically associated with a poor outcome [13, 14]. Our patient was anhepatic for a total of 10 hours, 3 hours in the operating, and 7 hours while waiting for a new liver in the ICU.
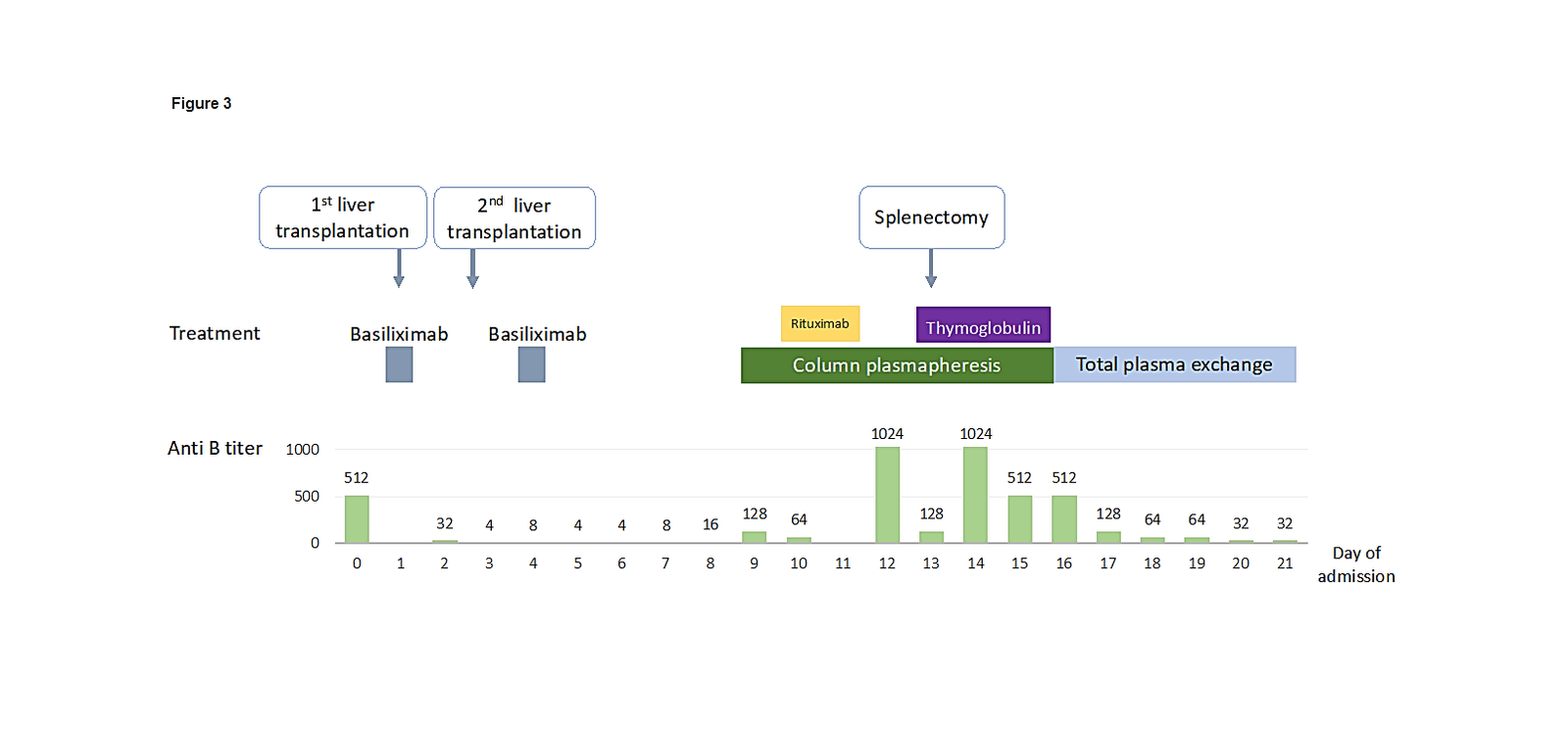
As in many other countries, there is a chronic shortage of organs available for transplantation. In these circumstances, crossing the ABO blood type barrier becomes the only chance for some patients to receive a new liver [15]. Since 2003 and the introduction of the Rituximab (anti -CD20 monoclonal antibody), the survival rate of ABO-Incompatible transplants has improved greatly. It remains a high-risk procedure, because of the possibilities of antibodies mediated rejection, hepatic arterial thrombosis and biliary complications. Our patient received Rituximab on day 9 and column plasmapheresis (immunoadsorption column) was started as well to prevent antibody-mediated graft rejection. The new ABO immunosorbent (IA), is the preferred technique to reduce the iso-titer. Because the IA-column is highly selective for anti-A/B antibodies, other antibodies are not affected, and no replacement fluid is required. In addition to antibody reduction capacity, the columns have a high capacity for ABO antibody removal without activation of coagulation factors, low activation of complement, no immune complex formation and no cytotoxicity [16].
Figure 3: Correspond to the ABO Incompatibility second liver transplantation. It shows the correlation between the beginning of column plasmapheresis treatment and the reduction of the anti B titer. Note also the use of Rituximab and Thymoglobulin that may have contribute to the decreased of the anti-B level.
A significant reduction of the titers of anti-B after 7 days of treatment (from 1024 to 512) was noted (Figure 3). However, a high titer of anti B was still persistent beside the specific plasmapheresis columns and the immunosuppression therapy, and a splenectomy salvage was performed in order to decrease the level of anti-B, leukocytosis and bilirubinemia. Most Asian centers use protocols with splenectomy in addition to other immunosuppressive measures [17]. Others did not observe any immunological advantage in ABO-I liver transplantation with no statistically significant differences in anti-A/B IgM and anti-A/B IgG titers between “splenectomy” and “non-splenectomy” groups [18]. In our patient, after the salvage splenectomy, the leukocytes count decreased significantly, as well as anti-B titer, as seen in the (Figure 3).
Conclusion
We describe the case of a 39-year-old patient, after an elective OLT that developed a fulminant hepatic failure. Our experience suggests that a multimodal therapy including transplant hepatectomy, prolonged anhepatic phase, plasmapheresis, and retransplantation of an even non-ABO compatible liver may result in a successful outcome of patients with acute fulminant liver failure complicating OLT. Twenty-one days after the first liver transplantation, the patient was discharged to a step-down ward, with no further requirement for ventilatory, hemodynamic or renal support.
Ethical Approval and Consent to Participate
Not applicable.
Consent
All the authors agree with the publication.
Conflicts of Interest
None.
Funding
None.
Acknowledgements
We would like to acknowledge Professor Cohen Jonathan for his contribution and precious help in building the case report.
Author Contributions
Olivier Zerbib: Responsible for practical performance and preparation of the case study report and review of literature.
David Dahan: Participated in writing the case study report and review of the literature.
Sornwichate Rattanachaiwong: Participated in writing the report and building the graphics.
Evyatar Nesher: Transplant surgeon and participated in review of the study report.
Marius Braun: Hepatologist, participated in writing and correcting the report.
Benjamin Zribi: Participated in writing the case study report and review of literature.
Liran Statlender: Participated in correcting the case study report and establishing the graphics.
Vered Yahalom: Specialist of transfusion, participated in elaborating column plasmapheresis.
Jonathan Cohen: Practical performance, participated in correcting the case study report and the performance review of the literature.
Pierre Singer: Responsible for the ICU Department, practical performance and review of literature for the case study report.
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Mon 11, May 2020Accepted: Thu 28, May 2020
Published: Thu 04, Jun 2020
Copyright
© 2023 Olivier Zerbib. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2020.01.08
Author Info
Benjamin Zribi David Dahan Eviatar Nesher Jonathan Cohen Liran Statlender Marius Braun Olivier Zerbib Pierre Singer Sornwichate Rattanachaiwong Vered Yahalom
Corresponding Author
Olivier ZerbibDepartment of General Intensive Care, Rabin Medical Center, Petah Tikva, Israel
Figures & Tables
References
- Alexander J C Ijtsma, Christian S van der Hilst, Marieke T de Boer, Koert P de Jong, Paul M J G Peeters et al. (2009) The clinical relevance of the anhepatic phase during liver transplantation. Liver Transpl 15: 1050-1055. [Crossref]
- S Emre, V Umman (2011) Split liver transplantation: an overview. Transplant Proc 43: 884-887. [Crossref]
- Tullio Piardi, Martin Lhuaire, Onorina Bruno, Riccardo Memeo, Patrick Pessaux et al. (2016) Vascular complications following liver transplantation: A literature review of advances in 2015. World J Hepatol 8: 36-57. [Crossref]
- H khalaf (2010) Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc 42: 865-870. [Crossref]
- Harendra Arora, Janine Thekkekandam, Leora Tesche, Raeshell Sweeting, David A Gerber et al. (2010) Long-term survival after 67 hours of anhepatic state due to primary liver allograft nonfunction. Liver Transpl 16: 1428-1433. [Crossref]
- Michael J Guirl, Jeffrey S Weinstein, Robert M Goldstein, Marlon F Levy, Goran B Klintmalm (2004) Two-stage total hepatectomy and liver transplantation for acute deterioration of chronic liver disease: a new bridge to transplantation. Liver Transpl 10: 564-570. [Crossref]
- Ubbo F Wiersema, Susan W Kim, David Roxby, Andrew Holt (2015) Therapeutic plasma exchange does not reduce vasopressor requirement in severe acute liver failure: a retrospective case series. BMC Anesthesiol 15: 30. [Crossref]
- Fin Stolze Larsen, Lars Ebbe Schmidt, Christine Bernsmeier, Allan Rasmussen, Helena Isoniemi et al. (2016) High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol 64: 69-78. [Crossref]
- Ya-Lien Cheng, Chih-Hsiang Chang, Wei-Ting Chen, Ming-Hung Tsai, Wei-Chen Lee et al. (2018) Prognostic factors and treatment effect of standard-volume plasma exchange for acute and acute-on-chronic liver failure: A single-center retrospective study. Transfus Apher Sci 57: 537-543. [Crossref]
- B Ringe, N Lübbe, E Kuse, U Frei, R Pichlmayr (1993) Total hepatectomy and liver transplantation as two-stage procedure. Ann Surg 218: 3-9. [Crossref]
- Bentdal, I B Brekke, M Olausson, A Foss, B Husberg et al. (2001) Living related liver retransplantation in a 6-month-old child after 60 hours of anhepatic phase following hepatectomy of thrombosed primary liver graft. Transplant Proc 33: 2497-2498. [Crossref]
- K J Oldhafer, A Bornscheuer, N R Frühauf, M K Frerker, H J Schlitt et al. (1999) Rescue hepatectomy for initial graft non-function after liver transplantation. Transplantation 67: 1024-1028. [Crossref]
- A Rana, M A Hardy, K J Halazun, D C Woodland, L E Ratner et al. (2008) Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transpl 8: 2537-2546. [Crossref]
- P A Clavien, P R Harvey, S M Strasberg (1992) Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation 53: 957-978. [Crossref]
- Silke Rummler, Astrid Bauschke, Erik Bärthel, Heike Jütte, Katrin Maier et al. (2016) Current techniques for AB0-incompatible living donor liver transplantation. World J Transplant 6: 548-555. [Crossref]
- Markus Wahrmann, Martin Schiemann, Lena Marinova, Günther F Körmöczi, Kurt Derfler et al. (2012) Anti-A/B antibody depletion by semiselective versus ABO blood group-specific immunoadsorption. Nephrol Dial Transplant 27: 2122-2129. [Crossref]
- SG Lee (2015) A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 15: 17-38. [Crossref]
- Vikram Raut, Akira Mori, Toshimi Kaido, Yasuhiro Ogura, Iida Taku et al. (2012) Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation 93: 99-105. [Crossref]
