Journals
Proline, A Peculiar Amino Acid with Astucious Functions in Development and Salt Tolerance Process in Plants
A B S T R A C T
Proline is known to play diverse functions in plants. Some aspects of its biological functions are still unclear. This review highlights some cases in the proline, structure, metabolism, functions in development and also its involvement in salt tolerance process in planta. Indeed, we report the clever roles of proline in cellular homeostasis, including redox balance and their implication as effector during some causal enzymological and physiological processes. Furthermore, the proline functions under abiotic stresses are not yet completely understood. The engineering of proline metabolism could lead to new opportunities to improve plant tolerance against environmental stresses, especially salinity. Eventually, we note that the purpose through this review is to provide a rich, concise and mostly cohesive source on proline, considered as a platform and an anchor between several disciplines and biological functions. We also provide insight on some important research gaps that need to be filled to advance our scientific understanding in this area of research on proline in soil-plant systems.
Keywords
Abiotic stress, plant development, proline metabolism, P5CS1, signal transduction
Introduction
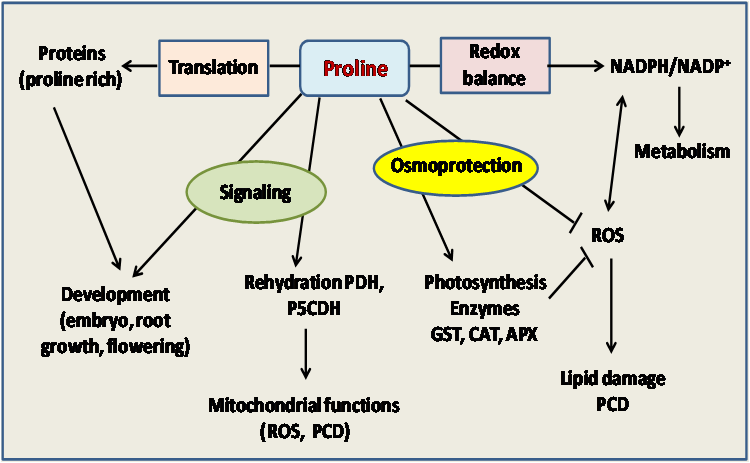
Proline is an amino acid with pivotal role in plant responses to environmental constraints [1-4]. Their amendment positively affected plant water relations, due mostly to enhancement in osmoregulation, as certain genotypes with greater mean leaf proline concentration and relative water content appeared to produce greater above-ground dry mass, when exposed to the external proline [4-7]. Indeed, exogenous proline resulted in significant increases in various phytometabolites such as polyphenol, carotenoids, chlorophyll, proline, carbohydrates, essential oil amounts and relative water content [3, 7, 8]. This amino acid has distinctive position among the proteinogenic amino acids. Many plants accumulate high levels of free proline in response to osmotic stress, that is generally known a protector or stabilizer of enzymes or membrane structures under susceptible dehydration or ionic induced stresses [6]. As a molecular chaperone, proline has also been demonstrated to protect protein integrity and increase the enzyme activities [3, 6, 9]. Proline is also suggested to bear an antioxidant role, having ROS scavenging activity and singlet oxygen quenching capacity [10, 11]. Eventually, the multifunctionality of proline may be summarized as followed in (Figure 1).
Proline Definition and Structure
Proline is one of the 20 DNA-encoded amino acids. This atypical residue is a non-essential amino acid and is defined, in genetic code, by fore codons which are the following: CCU, CCC, CCA and CCG. Proline is the sole among the 20 protein-forming amino acids in that the amine nitrogen is bound to two alkyl groups, thus making it a secondary amine [2, 3]. In addition, proline is widely recognized as playing an atypical role in the folding/unfolding transitions of the globular protein [2, 12]. Poverty or near absence of proline in the protein primary sequence may encodes their thermodynamic structure [13, 14]. At the same case, it is crucial to note that proline follows an atypical Ramachandran plot. This fact is due to the ring formation connected to the β-carbon; the ψ and φ angles about the peptide bond have less allowable degrees of rotation. The repercussion related to the proline intrinsically properties is often found in ‘turns’ of proteins. Indeed, the proline-free entropy is not as comparatively large to other amino acids. Hence, in a folded form vs. unfolded form, the change in entropy is less [15]. Furthermore, proline is rarely found in α and β structures as it would reduce the stability of such structures because its side chain α-N can only form one hydrogen bond [16-18]. In another hand, proline is the only amino acid that does not form a blue/purple color when developed by spraying with ninhydrin for uses in chromatography. Proline, instead, produces an orange/yellow color [2, 19, 20].
Figure 1: Multifunctional roles of proline in plants to mitigate deleterious effects of salinity stress as well as in protein synthesis and plant development.
APX: ascorbate peroxidase; CAT: catalase; PCD: programmed cell death.
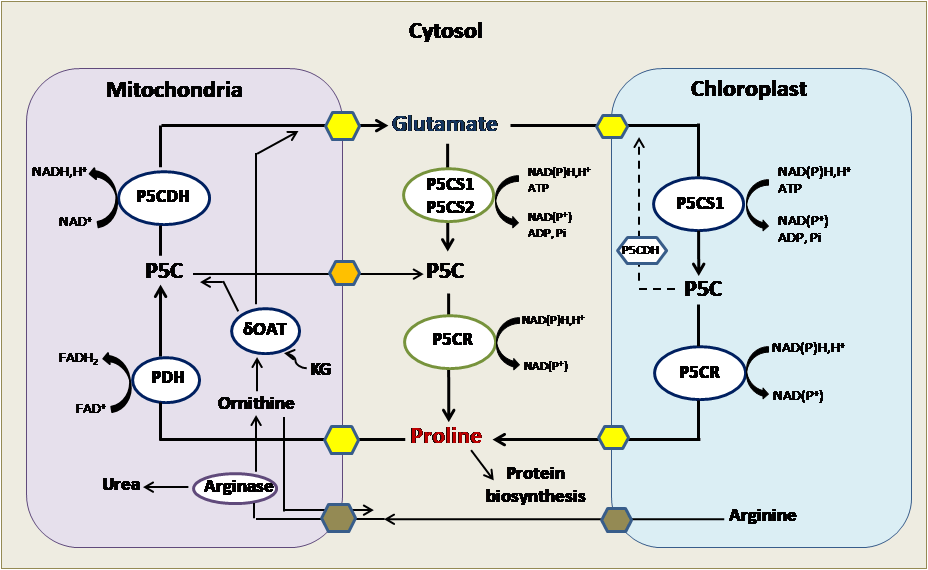
Figure 2: Proline metabolism in plants: Proline synthesis occurs in the cytosol and chloroplast. Proline degradation occurs in mitochondria.
P5C: δ-pyrroline-5-carboxylate; P5CR: pyrroline-5-carboxylate reductase; P5CS: pyrroline-5-carboxylate synthase; PDH: Proline dehydrogenase; P5CDH: Delta-1-pyrroline-5-carboxylate dehydrogenase; OAT: Ornithine aminotransferase; KG: α-ketoglutarate.
Proline Metabolism in Plant
In the vegetable context and precisely in plants, this peculiar residue is anabolized mainly from glutamate as indicated in (Figure 2), which is reduced to glutamate-semialdehyde (GSA) by the pyrroline-5-carboxylate synthetase (P5CS) and spontaneously converted to pyrroline-5-carboxylate (P5C) [2]. P5C reductase (P5CR) further reduces the P5C intermediate to proline. In most plant species, P5CS is encoded by two distinguished genes and P5CR is encoded by one gene. Proline catabolism occurs in mitochondria via the sequential action of proline dehydrogenase or proline oxidase (PDH or POX) producing P5C from proline, and P5C dehydrogenase (P5CDH), which converts P5C to glutamate. PDH is encoded by two genes, whereas a single P5CDH gene has been identified in Arabidopsis and tobacco. As an alternative pathway, proline can be synthesized from ornithine, which is transaminated first by ornithine-delta-aminotransferase (OAT) producing GSA and P5C, which is then converted to proline [2-4, 15, 21].
Intracellular proline level is encoded by the anabolism, the translocation between cells and different cellular compartments and the catabolism. Computer predictions suggest a mainly cytosolic localization of the biosynthetic enzymes (P5CS1, P5CS2 and P5CR), whereas a mitochondrial localization is predicted for the enzymes involved in proline catabolism, such as PDH1/ERD5, PDH2, P5CDH and OAT [21]. In addition, the signal peptides could not be identified within the primary structure of P5CS1, P5CS2 and P5CR enzymes, the PDH1, P5CDH and OAT proteins have well recognizable mitochondrial targeting signals [2, 4, 22].
We note also that P5CS1-GFP is normally localized in the cytosol of cell leaf mesophyll, but in embryonic cells and roots, it is associated with organelles that are like fusiform bodies [23]. When cells are exposed to salt or osmotic stress, P5CS1-GFP, but not P5CS2-GFP, accumulates in the chloroplasts. GFP-labeled Arabidopsis P5CS2 has been shown to be predominantly localized in the cytosol. The P5CR protein and activity has been detected in the cytosol and plastid fraction of leaf, root, and nodule cells of soybean. In pea mesophyll protoplasts, P5CR activity was localized in chloroplasts, suggesting that P5CR accumulates in plastids under high osmotic conditions [24]. Housekeeping proline anabolism probably occurs in the cytosol in Arabidopsis and it is controlled by the P5CS2 gene. During osmotic stress, proline anabolism is increased in the chloroplasts, which is controlled by the stress induced P5CS1 gene in Arabidopsis [25]. Then, proline can be anabolized in different subcellular compartments; this fact depends on the environmental conditions.
PDH and P5CDH are mitochondrial enzymes that use FAD and NAD+ as electron acceptors and generate FADH2 and NADH, respectively, delivering electrons for mitochondrial respiration. Recently, a P5C-proline cycle has been described proving that P5C, produced from proline in the mitochondria, can be transported into the cytosol and reduced to proline by cytosolic P5CR [26, 27]. When P5CDH activity is limited, the P5C-proline cycle can transfer more electrons to the mitochondrial electron transport chain and generate reactive oxygen species. P5CDH has been found in chloroplasts by proteome analysis, suggesting that P5C is also converted to glutamate in plastids [2, 4, 15].
At the same case, the ornithine pathway has been suggested to be more important as known (in conventional physiological conditions) during the development of seedling and in some plants for stress-induced proline accumulation [28]. Recently, the significance of the pathway and OAT in proline biosynthesis has been questioned because proline levels were not affected in Arabidopsis OAT knockout mutants. Instead, OAT facilitates nitrogen recycling from arginine through P5C that is converted to glutamate by P5CDH [3, 29].
Compartmentalization of proline metabolism implies that extensive intracellular proline transport must occur between the cytosol, chloroplasts and mitochondria. Physiological investigations suggest that proline uptake into mitochondria is an active process, hinting at the existence of specific amino acid transporters [2, 4]. Plasma membrane proline transporters, identified in several plants, mediate proline transport between cells and organs, but are not involved in organellar transport. Two proline carriers have recently been identified in the mitochondria of durum wheat: a proline uniporter, which facilitates proline transport into the mitochondrial matrix, and a proline/glutamate antiporter, which appears to have an important role in the Pro/Glu shuttle between the mitochondrial matrix and the cytosol [30]. Basic amino acid transporters (BAC) can deliver arginine and ornithine through the mitochondrial membrane. In the halophyte species Limonium latifolium, proline was sequestered to vacuoles in non-stressed plants, whereas in salt-stressed plants, high proline content was detected in the cytosol, suggesting the importance of “de novo proline biosynthesis” as well as transport for proline accumulation [21].
Proline Accumulation and Stress Tolerance
For a long time, proline was considered as an inert compatible osmolyte that protects subcellular macromolecules and structures under osmotic stress. However, proline accumulation can influence stress tolerance in multiple ways [4]. Proline has been shown to function as a molecular chaperone able to protect membrane protein integrity and enhance the activities of different enzymes. Examples of such roles include the prevention of protein aggregation and stabilization of M4 lactate dehydrogenase during extreme temperatures, protection of nitrate reductase during heavy metal and osmotic stress, and stabilization of ribonucleases and proteases upon arsenate exposure [31].
Exogenous Proline Encodes the Low Oxidative Damage and Favorable Ionic Homeostasis Under Salt Tolerance Condition
We note that proline was found to alleviate the salt damage to plant growth, a response closely related to reduced Na+ and Cl- accumulation together with increased K+ content [32, 33]. Also, exogenous proline efficiently upgrades the ROS scavenging system by enhancing the catalase, ascorbate peroxidase, and superoxide dismutase activities and the level of reduced ascorbate and glutathione [34, 35]. In addition, proline-sprayed stressed plants displayed a greater accumulation of this amino acid that was correlated with reduced Δ¹-pyrroline-5-carboxylate synthetase activity and increased proline dehydrogenase one. Consequently, proline-sprayed stressed plants showed decreases in H2O2 content and lipid peroxidation compared to only stressed plants. Our findings clearly evidence that proline treatment results in favorable changes in inorganic solute content and ROS scavenging system, reducing salt-induced oxidative damage and improving salt acclimation in maize plants. Hence, we can conclude that foliar spraying with proline was effective in reducing the deleterious effects of salinity. Indeed, the exogenous proline seems to act by modifying the toxic ion and organic solute content. Additionally, the proline contribution was basic in reducing oxidative damage by modulating the enzymatic and non-enzymatic antioxidant systems [36].
Regulatory Functions of Proline
It is crucial to note that various studies and investigations suggest that proline has certain regulatory functions like controlling plant development and acting as a signal molecule. Antisense expression of P5CS1 in transgenic Arabidopsis led to proline depletion and resulted in abnormal leaf morphology and defective inflorescences [25, 37]. A P5CS2-knockout mutant displays embryo lethality and proline-rescued mutant plants show aberrant growth, implying that an adequate proline supply is essential for embryo and plant development. P5CS2 and proline were suggested to regulate cell division and embryogenesis [38]. Overexpression of P5CS1 and enhanced proline content give birth to the early flowering of transgenic Arabidopsis plants, whereas the P5CS1 mutant with reduced proline content showed late flowering. Promotion of flower transition by CONSTANS can be partially mediated by activation of P5CS2 transcription and local rise of proline content.
Although proline is usually considered to be a metabolite with protective functions, several reports show that, under certain conditions, exogenous proline can be deleterious to plants and can inhibit growth and cell division. Proline treatment inhibited Arabidopsis seed germination, restricted growth of Petunia plants and arrested root growth of Thellungiella halophila, a plant with low PDH activity [4]. Externally added proline was more toxic to transgenic plants expressing antisense PDH as well as to PDH Arabidopsis mutants than to wild-type plants. In such plants, feedback inhibition of P5CS enzyme by proline might block proline biosynthesis and affect the NADP+/NADPH ratio and redox balance in plastids, leading to accelerated chlorophyll damage. Transcript profiling in Arabidopsis has revealed that one third of rehydration-inducible plant genes can also be induced by proline.
Up to 21 rehydration- and proline-inducible genes have been identified in Arabidopsis, most of which have the conserved PRE cis-acting element in their promoter regions, which is a target of specific bZIP-type transcriptional activators [2, 4, 39]. In yeast, proline can interact with the Put3p transcription factor and convert it from a transcriptionally inactive to an active form [40]. Active PUT3P promotes the expression of specific genes, including those that control proline catabolism. Some of the proline-activated genes can be important for stress adaptation. Uncoupled induction of PDH and P5CDH in P5CDH mutants leads to P5C and ROS accumulation, which can function as stress signal and cause programmed cell death. Proline metabolism can also influence programmed cell death in plants. In Arabidopsis, incompatible plant–pathogen interactions trigger a HR via ROS signals, which is accompanied by local activation of P5CS2 and proline accumulation [41, 42]. Proline can enhance PDH expression in such cells and lead to P5C accumulation, which in combination with ROS can function as an apoptotic signal and trigger HR during infection with avirulent pathogens. In flax, virulent pathogen infection was shown to activate the rust-inducible gene from flax (FIS1) gene, which encodes P5CDH [26, 43].
Although the exact role of FIS1 activation in virulent infection remains unclear, removal of P5C by FIS1/P5CDH during pathogen attack can reduce P5C-derived signals, diminish ROS levels and, therefore, suppress defenses, such as HR. Proline was recently proposed to modulate the plant defense response to Agrobacterium tumefaciens. Proline accumulates in plant tumors, and functions as a competitive antagonist of gamma-aminobutyric (GABA)-dependent plant defense, interfering with the GABA-induced degradation of quorum-sensing signal [44-46]. Proline can therefore promote Agrobacterium infection and horizontal transfer of the Ti plasmid. The above-listed examples suggest that proline functions as versatile cellular signal in both animal and plant cells [44, 45].
Suppression of Apoptosis by Proline in Fungal Pathogens and Other Species
ROS are known to play a role in cell communication and control of gene expression. Production of ROS in excess amounts can damage cells and lead to death also. It has been noticed that in Colletotrichum trifolii, a fungal pathogen of Medicago sativa, the mutationally activated oncogenic fungal small GTP-binding protein Ras (denoted as DARas) enhances the levels of ROS [3]. This causes abnormal fungal growth and development and ultimately apoptotic-like cell death but only when grown under nutrient-limiting conditions. This indicates that this fungal ras gene has the genetic capability to function as a bona fide oncogene. Very remarkably, restoration of the fungus to the wild-type phenotype requires only proline. Various studies described the ability of proline as a potent antioxidant and inhibitor of programmed cell death and also illustrated the effective quenching of ROS levels when proline was added to DARas mutant cells. Proline could also protect the wild type C. trifolii cells against different stresses like UV light, salt, heat and hydrogen peroxide [47, 48]. Therefore, these observations suggest that proline can scavenge intracellular ROS and also inhibit ROS-mediated apoptosis in fungal cells.
Proline in Floral Nectars
Pollinators are often attracted towards plants since they offer metabolically rich floral nectar. Amino acids such as proline occur in both extrafloral and floral nectars besides sugars, but the role of these amino acids in attracting the pollinators is not completely clear. It has been found that nectars of butterfly-pollinated flowers tend to have a higher content of amino acids than the flowers that are pollinated by bees and other animals [3, 49]. This finding suggests that amino acids are important attractants of butterflies to flowers. When both honeybees (Apis mellifera) and cabbage butterflies (Pieris rapae) were allowed to feed from artificial flowers containing sugar only or sugar plus amino acids, female cabbage white butterflies consumed more sugar-amino acid nectars, but male cabbage white butterflies could not discriminate between them [3, 50]. It appears that insect pollinators utilize proline preferentially during the initial phases of insect flight and can taste proline.
Various studies determined whether honeybees show any preference for synthetic nectar rich in proline. They clearly demonstrated that honeybees and other insects prefer nectars rich in proline and hypothesized that plants offer proline-rich nectars as a mechanism to attract pollinators. In addition, analysis of the proline content in ornamental tobacco (LxS8 line) flowers, which was 2020 μM, while the concentration of other amino acids is in the range of only 114 to 547 μM. Bertazzini et al. found that artificial nectar containing proline is preferred by forager honeybees [51]. While nectar containing alanine was preferred on the first day, serine was not at all preferred. Thus, bees clearly preferred proline above both alanine and serine, perhaps to use proline as a source of energy during flight. Proline may also play a role in taste and egg laying in honeybees [3].
Genetic Manipulation of Proline Metabolism
Proline biosynthetic pathway genes (P5CS1, P5CS2, P5CR) as well as genes associated with its degradation have been shown to be up and down regulated to validate their role during different abiotic stress conditions. Several reports have successfully showed increased tolerance to salinity, drought or cold stress by integrating proline biosynthetic pathway genes in several of the plant species. The list of transgenic plants developed for abiotic stress tolerance is shown in (Table 1). Initial attempts were made by Kavi Kishor et al. for overexpression of P5CS and generation of transgenic plants that overproduce proline [52]. The authors overexpressed a moth bean P5CS gene in tobacco plants, and the resultant transgenics synthesized 10 to 18-fold more proline than control plants and were more salt tolerant.
However, P5CS, being a rate-limiting enzyme in proline biosynthesis, is subjected to feedback inhibition by proline, and earlier reports suggested that proline accumulation in plants under stress might involve the loss of feedback regulation due to a conformational change in the P5CS protein. Hmida-Sayari et al. successfully engineered potato plants with the Arabidopsis P5CS gene under a stress-inducible promoter [53]. The transgenic potato cultivars accumulated high proline content in 100 mM NaCl stress and showed enhanced tuber yield and biomass in comparison with its wild type. Kumar et al. overexpressed the mutated P5CS129A gene in rice cultivars [54]. The transgenic rice variety showed enhanced proline accumulation, growth, and biomass. They were also characterized by low lipid peroxidation levels under salt stress (150 mM NaCl concentration).
Table 1: Transgenic plants developed using proline biosynthetic genes.
|
Gene |
Species |
Phenotypic effects of transgenic plants |
Reference |
|
Pyrroline-5-carboxylate Synthetase (P5CS) |
Rice |
Transgenic rice plants showed better root growth and biomass development during 200 mM NaCl treatment |
[65] |
|
P5CS |
Rice |
Reduced oxidative stress under osmotic stress |
[66] |
|
P5CS |
Wheat |
Wheat transgenic plants showed enhanced proline levels and conferred salt tolerance |
[67] |
|
P5CS |
Tobacco |
P5CS product levels between control and water tolerated plants indicated an increase of proline under normal irrigation and under drought stress conditions |
[68] |
|
P5CS |
Arabidopsis thaliana |
Antisense plants showed hypersensitivity to osmotic stress and show morphological changes during non-stress condition |
[69] |
|
P5CS |
Transgenic tobacco plant |
Significant increase in chlorophyll, fresh weight, dry weight and carbohydrate contents in transgenic compared to the non-transgenic plants |
[70] |
|
P5CS |
Tobacco (Nicotiana tabacum cv. Wisconsin) |
Overexpression of the P5CS gene in tobacco plants and consequent proline accumulation along with alleviation of CAT and APX activities increase drought tolerance in tobacco plants |
[71] |
|
P5CS cDNA from Arabidopsis thaliana |
Potato |
Transgenic plants showed an enhanced accumulation of proline in presence of salt and also showed much less altered tuber yield and weight compared to the non-transgenic ones |
[53] |
|
P5CS129A |
Rice (indica) |
Transgenic (T1) plants showed enhanced level of proline under 150 mM NaCl stress and better biomass production and growth performance under salt stress |
[54] |
|
Vigna cDNA P5CS |
Indica rice cultivar ADT 43 |
Transgenic plants grew well in the presence of 200 mM NaCl, while control plants died within 10 days following treatment |
[57] |
|
OsP5CS1 and OsP5CS2 from Rice |
Tobacco |
Enhanced 3.2 times proline content, biomass production oxidative stress protection. |
[55] |
|
AtP5CS1 |
Arabidopsis thaliana |
Proline accumulation under heat stress decreases the thermotolerance, probably by increased ROS production via the Pro/P5C cycle and inhibition of ABA and ethylene biosynthesis |
[27] |
|
Pyrroline- 5-carboxylate Reductase (P5CR) |
Soybean |
Enhanced heat and drought stress |
[72] |
|
P5CR |
Tobacco |
Enhanced P5CR activity in transgenics did not yield signifi cant increase in proline level |
[73] |
|
P5CR |
Soybean |
Antisense plants produced low number of seeds |
[72] |
|
Proline dehydrogenase |
Arabidopsis thaliana |
Altered levels of proline dehydrogenase conferred salt and freezing tolerance |
[69] |
|
Proline dehydrogenase |
Tobacco |
Antisense plants showed increased proline content |
[74] |
|
Ornithine-δ- aminotransferase |
Tobacco |
Overexpression increased proline biosynthesis and osmotolerance |
[75] |
|
Ornithine-δ- aminotransferase |
Rice |
Overexpression increased proline 5–15 fold of that in non-transgenic control plants during osmotic stress and transgenic plants showed improved yield under stress conditions |
[76] |
In another study, Zhang et al. co-expressed rice OsP5CS1 and OsP5CS2 genes in tobacco [55]. The second-generation transgenic plants exhibited 2.3 times more root length, 3.2 times proline content and 3.9 times average fresh weight when compared with control cultivars under 200 mM NaCl stress. These results highlighted the importance of the P5CS enzymes in enhanced proline accumulation, as well as reduced cellular oxidative damage under the influence of the abiotic stress environment. A similar increase in proline production has been carried out exploiting the proline biosynthetic and degradation genes (P5CS, P5CR, OAT and P5CDH) in transgenic sugarcane, rice, wheat, olives, carrots, jerusalem artichokes, Medicago truncatula and sweet potatoes that confer tolerance to drought and salt stress [56-64].
Conclusions and Outlook
As a conclusion, it is basic to signal that in response to various environmental stresses, many plant species synthesize proline in the cytosol and accumulates in the chloroplasts. Proline accumulation in plants is a well-recognized physiological reaction to osmotic stress prompted by salinity, drought and other abiotic stresses. Indeed, proline plays an important role in plants through their protection against various stresses and also helps them to recover from those constraint’s more rapidly. At the same case and when applied exogenously to plants exposed to stress, proline results in increased growth and other physiological characteristics. In addition, exogenous proline scavenges the ROS generated in plants under various biotic and abiotic stresses. Hence, Proline plays several protective functions such as osmoprotectant, stabilizing cellular structures, enzymes, and scavenging ROS and keeps up redox balance in adverse situations. In addition, ample-studied osmoprotective capacity, proline has been also ensnared in the regulation of plant improvement, including flowering, pollen, embryo and leaf enlargement. Albeit, ample is now well-known about proline metabolism, but certain characteristics of its biological roles are still indistinct. Proline metabolism is directed by several cellular mechanisms, of which we identify only very fewer mechanisms. Furthermore, in the future, we will strive to identify such types of cellular mechanisms.
Acknowledgements
This study was supported by a grant from the Ministry of Higher Education and Scientific Research of Tunisia.
Conflicts of Interest
None.
Author Contributions
WS and FB wrote the manuscript and FB edited the final version of the manuscript.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 20, Jun 2020Accepted: Fri 10, Jul 2020
Published: Mon 31, Aug 2020
Copyright
© 2023 Faiçal Brini. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JFNM.2020.02.04
Author Info
Corresponding Author
Faiçal BriniBiotechnology and Plant Improvement Laboratory, Centre of Biotechnology of Sfax, University of Sfax, Sfax, Tunisia
Figures & Tables
Table 1: Transgenic plants developed using proline biosynthetic genes.
|
Gene |
Species |
Phenotypic effects of transgenic plants |
Reference |
|
Pyrroline-5-carboxylate Synthetase (P5CS) |
Rice |
Transgenic rice plants showed better root growth and biomass development during 200 mM NaCl treatment |
[65] |
|
P5CS |
Rice |
Reduced oxidative stress under osmotic stress |
[66] |
|
P5CS |
Wheat |
Wheat transgenic plants showed enhanced proline levels and conferred salt tolerance |
[67] |
|
P5CS |
Tobacco |
P5CS product levels between control and water tolerated plants indicated an increase of proline under normal irrigation and under drought stress conditions |
[68] |
|
P5CS |
Arabidopsis thaliana |
Antisense plants showed hypersensitivity to osmotic stress and show morphological changes during non-stress condition |
[69] |
|
P5CS |
Transgenic tobacco plant |
Significant increase in chlorophyll, fresh weight, dry weight and carbohydrate contents in transgenic compared to the non-transgenic plants |
[70] |
|
P5CS |
Tobacco (Nicotiana tabacum cv. Wisconsin) |
Overexpression of the P5CS gene in tobacco plants and consequent proline accumulation along with alleviation of CAT and APX activities increase drought tolerance in tobacco plants |
[71] |
|
P5CS cDNA from Arabidopsis thaliana |
Potato |
Transgenic plants showed an enhanced accumulation of proline in presence of salt and also showed much less altered tuber yield and weight compared to the non-transgenic ones |
[53] |
|
P5CS129A |
Rice (indica) |
Transgenic (T1) plants showed enhanced level of proline under 150 mM NaCl stress and better biomass production and growth performance under salt stress |
[54] |
|
Vigna cDNA P5CS |
Indica rice cultivar ADT 43 |
Transgenic plants grew well in the presence of 200 mM NaCl, while control plants died within 10 days following treatment |
[57] |
|
OsP5CS1 and OsP5CS2 from Rice |
Tobacco |
Enhanced 3.2 times proline content, biomass production oxidative stress protection. |
[55] |
|
AtP5CS1 |
Arabidopsis thaliana |
Proline accumulation under heat stress decreases the thermotolerance, probably by increased ROS production via the Pro/P5C cycle and inhibition of ABA and ethylene biosynthesis |
[27] |
|
Pyrroline- 5-carboxylate Reductase (P5CR) |
Soybean |
Enhanced heat and drought stress |
[72] |
|
P5CR |
Tobacco |
Enhanced P5CR activity in transgenics did not yield signifi cant increase in proline level |
[73] |
|
P5CR |
Soybean |
Antisense plants produced low number of seeds |
[72] |
|
Proline dehydrogenase |
Arabidopsis thaliana |
Altered levels of proline dehydrogenase conferred salt and freezing tolerance |
[69] |
|
Proline dehydrogenase |
Tobacco |
Antisense plants showed increased proline content |
[74] |
|
Ornithine-δ- aminotransferase |
Tobacco |
Overexpression increased proline biosynthesis and osmotolerance |
[75] |
|
Ornithine-δ- aminotransferase |
Rice |
Overexpression increased proline 5–15 fold of that in non-transgenic control plants during osmotic stress and transgenic plants showed improved yield under stress conditions |
[76] |
APX: ascorbate peroxidase; CAT: catalase; PCD: programmed cell death.
P5C: δ-pyrroline-5-carboxylate; P5CR: pyrroline-5-carboxylate reductase; P5CS: pyrroline-5-carboxylate synthase; PDH: Proline dehydrogenase; P5CDH: Delta-1-pyrroline-5-carboxylate dehydrogenase; OAT: Ornithine aminotransferase; KG: α-ketoglutarate.
References
- Saibi W, Feki K, Ben Mahmoud R, Brini F (2015) Durum wheat dehydrin (DHN-5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 242: 1187-1194. [Crossref]
- Saibi W, Feki K, Yacoubi I, Brini F (2015) Bridging between proline structure, functions, metabolism, and involvement in organism physiology. Appl Biochem Biotech 176: 2107-2119. [Crossref]
- Suprasanna P, Rai AN, HimaKumari P, Kumar SA, Kavi Kishor P (2014) Modulation of proline: implications in plant stress tolerance and development. Plant Adaptation to Environmental Change (eds NA Anjum, SS Gill and R. Gill) CABI Publishers UK 68-93.
- Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15: 89-97. [Crossref]
- Bouazzi H, Feki K, Brini F, Saibi W (2019) Is duality between proline metabolic mutation (p5cs 1-4) and durum wheat dehydrin transgenic contexts a “pacemaker” for salt tolerance process in Arabidopsis thaliana? Acta Physiol Plant 41: 36.
- Bouazzi H, Feki K, Zouari N, Sahnoun M, Brini F et al. (2019) Causal Enzymology and Physiological Aspects May Be Accountable to Membrane Integrity in Response to Salt Stress in Arabidopsis thaliana Lines. BioMed Res Int 2019: 3534943. [Crossref]
- Zali AG, Ehsanzadeh P (2018) Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind Crops Prod 111: 133-140.
- Saibi W, Zouari N, Masmoudi K, Brini F (2016) Role of the durum wheat dehydrin in the function of proteases conferring salinity tolerance in Arabidopsis thaliana transgenic lines. Int J Biol Macromol 85: 311-316. [Crossref]
- Saibi W, Drira M, Yacoubi I, Feki K, Brini F (2015) Empiric, structural and in silico findings give birth to plausible explanations for the multifunctionality of the wheat dehydrin (DHN-5). Acta Physiol Plant 37: 5.
- Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2: 53.
- Suprasanna P, Nikalje G, Rai A (2016) Osmolyte accumulation and implications in plant abiotic stress tolerance. In Osmolytes and plants acclimation to changing environment: Emerging omics technologies. Springer 1-12.
- Trovato M, Mattioli R, Costantino P (2008) Multiple roles of proline in plant stress tolerance and development. Rendiconti Lincei 19: 325-346.
- Uversky VN (2002) Natively unfolded proteins: a point where biology waits for physics. Protein Sci 11: 739-756. [Crossref]
- Yaron A, Naider F, Scharpe S (1993) Proline-dependent structural and biological properties of peptides and proteins. Crit Rev Biochem Mol Biol 28: 31-81. [Crossref]
- Saibi W, Abdeljalil S, Masmoudi K, Gargouri A (2012) Biocatalysts: beautiful creatures. Biochem Biophys Res Commun 426: 289-293. [Crossref]
- Chengcheng L (2012) New Methods to Study Proline-Rich Disordered Regions and Their Structural Ensembles in Protein Signaling Pathways. PhD Thesis.
- Mattoo RUH (2014) Mechanistic insights into cytosolic molecular chaperones in protein unfolding and disaggregation. Université de Lausanne, Faculté de biologie et médecine. PhD Thesis.
- Deepak RNVK, Sankararamakrishnan R (2016) Unconventional N-H…N Hydrogen Bonds Involving Proline Backbone Nitrogen in Protein Structures. Biophysic J 110: 1967-1979. [Crossref]
- Bhushan R, Reddy G (1987) TLC of phenylthiohydantoins of amino acids: a review. J Liq Chrom Rel Techn 10: 3497-3528.
- Tiwari A (2015) Practical Biochemistry: A Student Companion. LAP LAMBERT Acad Publish.
- Shafi A, Zahoor I, Mushtaq U (2019) Proline Accumulation and Oxidative Stress: Diverse Roles and Mechanism of Tolerance and Adaptation Under Salinity Stress. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches. Springer 269-300.
- Nahar K, Hasanuzzaman M, Fujita M (2016) Roles of osmolytes in plant adaptation to drought and salinity. In: Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies. Springer 37-68.
- Roychoudhury A, Banerjee A, Lahiri V (2015) Metabolic and molecular-genetic regulation of proline signaling and its cross-talk with major effectors mediates abiotic stress tolerance in plants. Turk J Bot 39: 887-910.
- Naliwajski MR, Skłodowska M (2014) Proline and its metabolism enzymes in cucumber cell cultures during acclimation to salinity. Protoplasma 251: 201-209. [Crossref]
- Székely G, Ábrahám E, Cséplő Á, Rigó G, Zsigmond L et al. (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53: 11-28. [Crossref]
- Qamar A, Mysore K, Senthil Kumar M (2015) Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front Plant Sci 6: 503. [Crossref]
- Lv WT, Lin B, Zhang M, Hua XJ (2011) Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol 156: 1921-1933. [Crossref]
- Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ 3: 89.
- Bringaud F, Barrett MP, Zilberstein D (2012) Multiple roles of proline transport and metabolism in trypanosomatids. Front Biosci 17: 349-374. [Crossref]
- Jarzyniak, KM, Jasiński M (2014) Membrane transporters and drought resistance-a complex issue. Front Plant Sci 5: 687. [Crossref]
- Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS et al. (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9: 285. [Crossref]
- Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59: 206-216.
- Nounjan N, Nghia PT, Theerakulpisut P (2012) Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol 169: 596-604. [Crossref]
- Hasanuzzaman M, Alam M, Rahman A, Hasanuzzaman MD, Nahar K et al. (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa) varieties. BioMed Res Inter 757219.
- Nahar K, Hasanuzzaman M, Alam M, Fujita M (2015) Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 7: plv069. [Crossref]
- Hossain MA, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In Oxidative damage to plants. Elsevier 477-522.
- Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63: 1593-1608. [Crossref]
- Mattioli R, Falasca G, Sabatini S, Altamura MM, Costantino P et al. (2009) The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plant 137: 72-85. [Crossref]
- Coraggio I, Tuberosa R (2004) Molecular Bases of Plant Adaptation to Abiotic Stress and Approaches to Enhance Tolerance to Hostile Environments. Handbook Plant Biotechnology 22.
- MacPherson S, Larochelle M, Turcotte B (2006) A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70: 583-604. [Crossref]
- Rejeb KB, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80: 278-284. [Crossref]
- Trovato M, Forlani G, Signorelli S, Funck D (2019) Proline Metabolism and Its Functions in Development and Stress Tolerance. In: Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants. Springer 41-72.
- Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Signal 19: 998-1011. [Crossref]
- Haudecoeur E, Planamente S, Cirou A, Tannieres M, Shelp B et al. (2009) Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Nat Acad Sci U S A 106: 14587-14592. [Crossref]
- Dessaux Y, Faure D (2018) Quorum sensing and quorum quenching in agrobacterium: a “go/no go system”? Genes (Basel) 9: 210. [Crossref]
- Monteoliva MI, Rizzi YS, Cecchini NM, Hajirezaei MR, Alvarez ME (2014) Context of action of proline dehydrogenase (ProDH) in the hypersensitive response of Arabidopsis. BMC Plant Biol 14: 21. [Crossref]
- Chen C, Dickman MB (2005) Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc Nat Acad Sci U S A 102: 3459-3464. [Crossref]
- Tanveer M, Shabala S (2018) Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory. Transport and Signaling Mechanisms 213.
- Romeis J, Wäckers F (2000) Feeding responses by female Pieris brassicae butterflies to carbohydrates and amino acids. Physiol Entomol 25: 247-253.
- Dötterl S, Vereecken N (2010) The chemical ecology and evolution of bee–flower interactions: a review and perspectives. Canad J Zool 88: 668-697.
- Bertazzini M, Medrzycki P, Bortolotti L, Maistrello L, Forlani G (2010) Amino acid content and nectar choice by forager honeybees (Apis mellifera). Amino Acids 39: 315-318. [Crossref]
- Kishor KPB, Hong Z, Miao GH, Hu C, Verma DPS (1995) Over expression of Δ1-pyrroline-5- carboxylate synthetase increases proline overproduction and confers osmotolerance in transgenic plants. Plant Physiol 108: 1387-1394. [Crossref]
- Hmida Sayari A, Gargouri Bouzid R, Bidani A, Jaoua L, Savoure A et al. (2005) Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169: 746-752.
- Kumar V, Shriram V, Kavi Kishor PB, Jawali N, Shitole MG (2010) Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotech Rep 4: 37-48.
- Zhang X, Tang W, Liu J, Liu Y (2014) Co-expression of rice OsP5CS1 and OsP5CS2 genes in transgenic tobacco resulted in elevated proline biosynthesis and enhanced abiotic stress tolerance. Chin J Appl Environ Biol 20: 717-722.
- Guerzoni JTS, Belintani NG, Moreira RMP, Ho Shimo AA, Domingues DS et al. (2014) Stress-induced Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene confers tolerance to salt stress in transgenic sugarcane. Acta Physiol Plant 36: 2309-2319.
- Karthikeyan A, Pandian SK, Ramesh M (2011) Transgenic indica rice cv. ADT 43 expressing a Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. PCTOC 107: 383-395.
- You J, Hu, H, Xiong L (2012) An ornithine-δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci 197: 59-69. [Crossref]
- Vendruscolo EG, Schuster I, Pileggi M, Scapimd CA, Molinari HBC et al. (2007) Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J Plant Physiol 164: 1367-1376. [Crossref]
- Behelgardy MF, Motamed N, Jazii FR (2012) Expression of the P5CS gene in transgenic versus non transgenic Olive (Olea europaea) under salinity stress. World Appl Sci J 18: 580-583.
- Han KH, Hwang CH (2003) Salt tolerance enhanced by transformation of a P5CS gene in carrot. J Plant Biotechnol 5: 149-153.
- Huang Z, Long Z, Chen D, Liang M, Liu Z et al. (2013) Salt Stress Encourages Proline Accumulation by Regulating Proline Biosynthesis and Degradation in Jerusalem Artichoke Plantlets. PLos One 8: e62085. [Crossref]
- Verdoy D, De La Pena TC, Redondo FJ, Lucas MM, Pueyo JJ (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ 29: 1913-1923. [Crossref]
- Liu D, He S, Zhai H, Wang L, Zhao YB et al. (2014) Overexpression of IbP5CR enhances salt tolerance in transgenic sweet potato. PCTOC 117: 1-16.
- Anoop N, Gupta AK (2003) Transgenic indica rice cv IR-50 over-expressing Vigna aconitifolia delta (1)-pyrroline-5-carboxylate synthetase cDNA shows tolerance to high salt. J Plant Biochem Biotechnol 12: 109-116.
- Hong Z, Lakkineni K, Zhang Z, Verma DP (2000) Removal of feedback inhibition of pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122: 1129-1136. [Crossref]
- Sawahel WA, Hassan AH (2002) Generation of transgenic wheat plants producing high levels of the osmoprotectant proline. Biotech Lett 24: 721-725.
- Yamchi A, Rastgar Jazii F, Karkhanehee AA, Ghobadi C, Mousavi A (2005) Increasing of tolerance to osmotic stresses in tobacco Nicotiana tabacum cv. Xanthi through overexpression of P5CS gene. J Agri Sci Technol 1: 251-264.
- Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi Shinozaki K et al. (1999) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett 461: 205-210. [Crossref]
- Mahboobeh R, Akbar EA (2013) Effect of salinity on growth, chlorophyll, carbohydrate and protein contents of transgenic Nicotiana plumbaginifolia over expressing P5CS gene. E3 J Environ Res Manage 4: 163-170.
- Zarei S, Ehsanpour AA, Abbaspour J (2012) The role of over expression of P5CS gene on proline, catalase, ascorbate peroxidase activity and lipid peroxidation of transgenic tobacco (Nicotiana tabacum) plant under in vitro drought stress. J Cell Mol Res 4: 43-49.
- De Ronde JA, Cress WA, Kruger GHJ, Strasser RJ, Van Staden J (2004) Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5VR gene, during heat and drought stress. J Plant Physiol 161: 1211-1224. [Crossref]
- LaRosa PC, Rhodes D, Rhodes JC, Bressan RA, Csonka LN (1991) Elevated accumulation of proline in NaCl-adapted tobacco cells is not due to altered Δ1-pyrroline-5-carboxylate reductase. Plant Physiol 96: 245-250. [Crossref]
- Kochetov AV, Titov SE, Kolodyazhnaya YS, Komarova ML, Koval VS et al. (2004) Tobacco transformants bearing antisense suppressor of proline dehydrogenase gene are characterized by higher proline content and cytoplasm osmotic pressure. Russ J Genet 40: 216-218.
- Roosens NH, Al Bitar F, Loenders K, Angenon G, Jacobs M (2002) Overexpression of ornithine-δ-aminotransferase increases proline biosynthesis and confers osmotolerance in transgenic plants. Mol Breeding 9: 73-80.
- Wu LQ, Fan ZM, Guo L, Li YQ, Zhang WJ et al. (2003) Over-expression of an Arabidopsis delta-OAT gene enhances salt and drought tolerance in transgenic rice. Chin Sci Bull 48: 2594-2600.
