Primary Thyroid Lymphoma in an Adolescent with Hashimoto’s Thyroiditis and Congenital Deafness
A B S T R A C T
Background: Primary thyroid lymphoma is extremely rare in children and adolescents. The diagnosis of thyroid malignancies is often made secondary to other thyroid pathologies including Hashimoto’s thyroiditis (HT).
Case Presentation: This case describes a 14-year-old girl with a background of HT and congenital deafness that presented with a rapidly enlarging multinodular thyroid mass. She underwent a total thyroidectomy and a diffuse, large B-cell lymphoma was diagnosed on pathology.
Discussion: Primary thyroid disease is an extremely rare disease in childhood, usually presenting as a rapidly enlarging goitre. The correlation between Hashimoto’s thyroiditis and primary thyroid lymphoma is well-established in adults but has not been verified in the paediatric population. Our patient also has β- thalassemia and Usher syndrome, however, no correlation between these and primary thyroid lymphoma could be found in the literature.
Conclusion: It is important to include malignancy in the differential diagnosis of thyroid enlargement or unexplained symptoms, especially in the presence of syndromes. Further research into the relation between lymphomas and HT in the paediatric population is needed.
Keywords
Thyroid, lymphoma, congenital deafness, thalassemia, Hashimoto’s thyroiditis, primary thyroid lymphoma, Usher syndrome, children, adolescents
Introduction
Primary thyroid lymphoma (PTL) in children and adolescents is an extremely rare malignancy of lymphocytic cells within the thyroid [1, 2]. Limited data regarding the incidence of PTL are available but has been reported to constitute 2-3% of all pediatric thyroid malignancies [3-5]. Hashimoto’s thyroiditis (HT) is a known risk factor for PTL in adults [6, 7]. In children the role of HT in the development of thyroid cancer is unclear with studies suggesting both no association and an increased risk [8, 9]. The diagnosis of a primary malignancy in the thyroid is often made secondary to another thyroid pathology for example malignant transformation of a HT related goitre [9-11]. Pediatric-type follicular lymphoma is a biologically distinct, but clinically rare subtype of follicular lymphoma in children [12, 13]. The diagnostic distinction is of importance when deciding upon a management plan regarding treatment intensity [12, 13].
This case describes an adolescent girl with congenital deafness who presented with Hashimoto’s thyroiditis that progressed to primary thyroid lymphoma. The discussion focusses on the contributing factors for the development and co-morbidities associated with PTL.
Case Presentation
A 14-year-old female with congenital deafness and HT from North African descent was referred to the pediatric oncology unit following an elective total thyroidectomy for an enlarging goitre. Histology of the thyroid revealed a PTL. She had been diagnosed with congenital bilateral sensorineural deafness as an infant for which she was fitted with a cochlear implant. The patient and her older male and younger female siblings, from consanguineous parents, were diagnosed with the same deafness. The father was diagnosed with hypothyroidism secondary to benign thyroid enlargement. Although he has no hearing impairment, he does suffer from loss of balance.
Although the patient was asymptomatic and euthyroid, Hashimoto’s thyroiditis was suspected based on the presence of a goitre and a hypoechogenic enlarged thyroid mass with moderate hypervascularisation on ultrasound. After two months HT was confirmed on the basis of tiredness, hypothyroidism and elevated anti-thyroid peroxidase antibodies. She had an initial clinical and biochemical response on L-thyroxine, before representing with tiredness and a painless, rapid enlarging goitre over the course of three months despite being euthyroid. The clinical examination confirmed a large, asymmetrical multinodular thyroid swelling without any lymphadenopathy or hepatosplenomegaly. No constitutional symptoms such as fever, night sweats or weight loss were present.
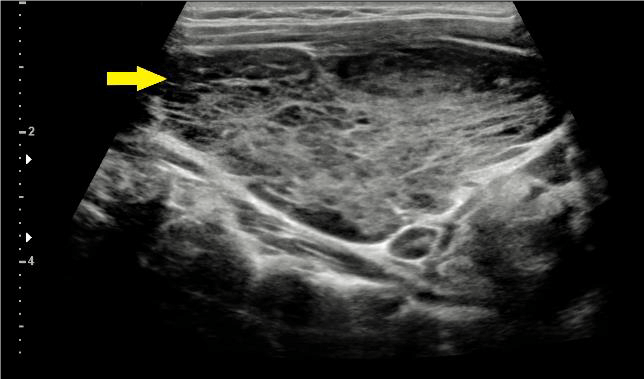
An ultrasound confirmed a marked enlarged goitre with a hypoechogenic parenchyma and a right lobe volume of 22 mL and left lobe volume of 110 mL (Figure 1). Computed tomography (CT) confirmed a multinodular goitre, particularly in the left lobe which measured 5.5 x 10 x 5.8 cm (Figure 2). Both ultrasound and CT demonstrated trachea deviation without any signs of obstruction. No reports indicated solitary, irregular nodules suggestive of malignant changes.
Figure 1: Diffuse enlarged thyroid gland (yellow arrow) with mildly increased vascularisation.
Figure 2: Computed tomography with a multinodular goitre of both lobes. Airway compression is indicated by the red arrow.
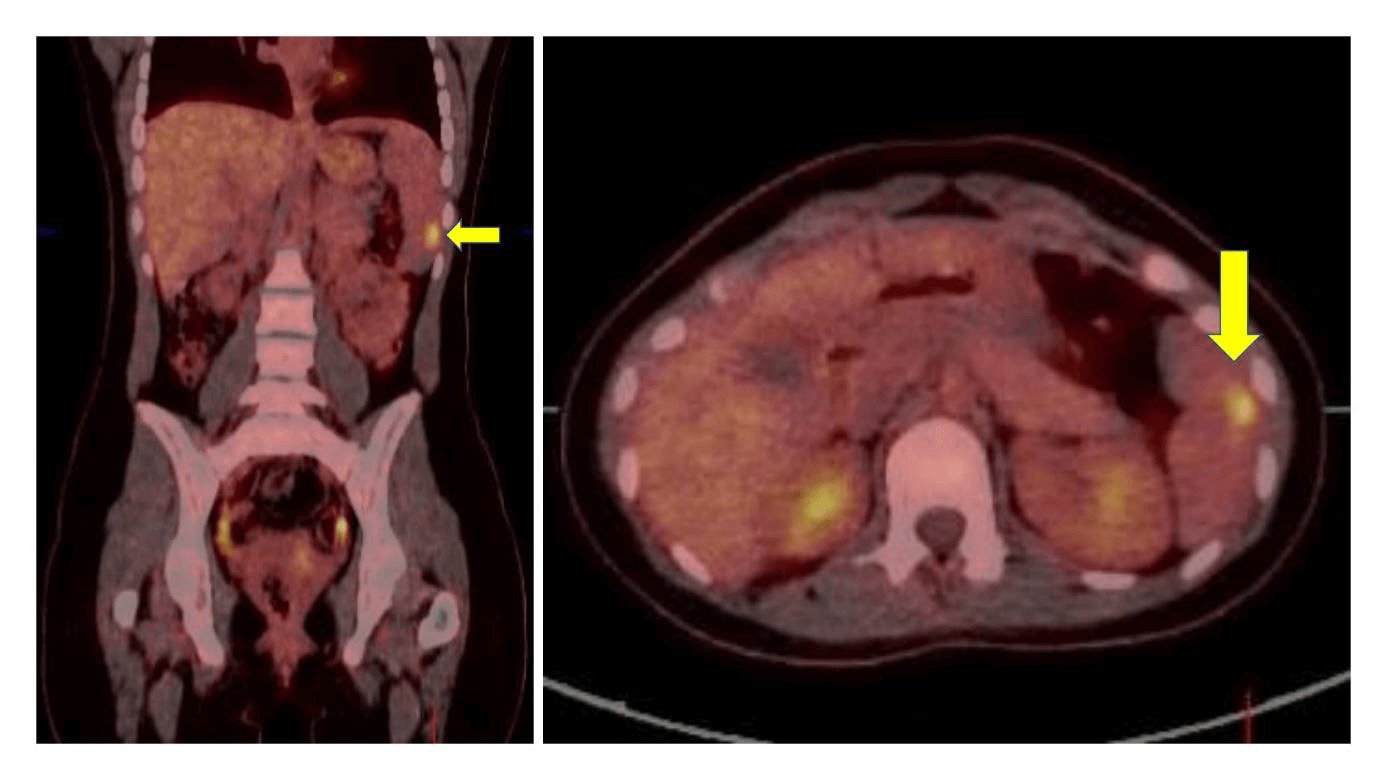
A total thyroidectomy was performed. The pathology examination of the thyroid revealed a multistage process involving evolution from chronic lymphocytic inflammation consistent with the diagnosis of HT to a pediatric type of follicular lymphoma to a diffuse large B-cell lymphoma (DLBCL) (Figure 3). A primary thyroid lymphoma stage IIE according to the Ann Arbor staging classification was diagnosed with a positron emission tomography- CT (PET-CT) revealing a right sided paratracheal pathological adenopathy measuring 16 mm and a malignant splenic nodule measuring 9mm (Figure 4); in the absence of pathological involvement on both bone marrow aspirate and trephine and lumbar puncture [11].
Figure 3: A-B) H&E staining of a histological section through the thyroid with complete obliteration of the normal follicular epithelial structures by a lymphocytic infiltrate, organised in either a follicular pattern (A-I) or in other area, a diffuse growth pattern (B-J). C) Immunohistochemical staining illustrating the high KI67 labelling index in the follicular structures. D) The diffuse areas are composed of medium-sized to large centroblasts. The immunophenotype confirms the transformation of a paediatric-type follicular lymphoma to a diffuse large B-cell lymphoma, with expression of CD20 (E-F) and CD10 in de lymphoid cells (G-H). Anti-CD21 immunostaining illustrates the presence and absence of underlying follicular dendritic cell networks in the follicular and diffuse components, respectively (I-J).
A mild microcytic anaemia with haemoglobin of 11.2 g/dL and MCV of 59.3fL; and iron deficiency was diagnosed prior to oncological treatment. She completed chemotherapy according to LMB-based recommendations for low/intermediate risk B-Non-Hodgkin Lymphoma (NHL), consisting of cyclophosphamide, vincristine, prednisone, doxorubicin, methotrexate, cytarabine and intrathecal methotrexate. The patient remains in full remission a year after completion of therapy. After the initial oncological diagnosis, but independent from the patient’s treatment, the patient’s family underwent oto-genetic screening for congenital deafness and thyroid dysfunction affecting two generations. Genetic testing revealed homozygote c.3122+1G>A variant in PCDH15, confirming the diagnosis of Usher Syndrome type 1 in both the patient and her brother.
Figure 4: The malignant splenic nodule measuring 9 mm detected on an FDG-PET/CT scan.
Despite confirmed iron repletion and regulated thyroid functions on replacement therapy before and during chemotherapy, the patient maintained a microcytic red cell morphology with haemoglobin in the normal range after completing chemotherapy. A haemoglobin electrophoresis confirmed a β- thalassemia minor/intermedia. Subsequently both her brothers were confirmed with β- thalassemia minor/intermedia.
Discussion
Thyroid malignancies continue to be rare in children and adolescents despite an increase in incidence over the past three decades [9]. Differentiated thyroid cancer is the most common accounting for 90-95% of all pediatric thyroid malignancies, while primary thyroid lymphoma (PTL) represents only 2-3% [5, 9]. Thyroid nodules are rarer in the pediatric population; however, they are more likely to be malignant than in adults [9, 10, 14].
PTL is defined as a lymphoma that arises from the thyroid gland, while secondary thyroid lymphoma originates from a disseminated non-thyroidal malignancy that metastases to the thyroid gland [7, 15]. PTL presents in general as a rapidly enlarging goitre or thyroid nodule in 70-80% of cases, in one-third of patients accompanied by compressive symptoms including dyspnoea, dysphagia, stridor, cough or hoarseness which was not present in this case [2, 6]. Systemic symptoms like weight loss, fever and nocturnal sweats are only present in approximately 10-20% of the patients [2, 6, 7]. Both primary and metastatic pathologies differ in treatment and clinical course, with PTL having a lower mortality rate in early stages [15]. PTL usually presents in the sixth or seventh decade of life affecting more women with a 2-8:1 female predominance [11]. The predominant type is B-cell NHL [2, 11]. The most common histological subtypes are Diffuse Large B Cell Lymphoma (DLBCL) and mucosa-associated lymphoid tissue (MALT) lymphoma which account for 50-70% and 10-23% of the cases respectively. Other less frequent subtypes include follicular, small lymphocytic and Hodgkin’s lymphoma; even rarer are Burkitt’s, T-cell, mantle cell and lymphoblastic lymphomas [7, 11]. DLBCL exhibits a more aggressive behaviour and subsequently has a worse prognosis than the more indolent MALT and follicular lymphomas [7, 11, 16]. A MALT lymphoma can secondarily transform into DLBCL resulting in a mixed type that behaves clinically like the DLBCL subtype [7, 17].
PTL arises from a malignant transformation of lymphoid cells after lymphoid tissue migrated into the thyroid during inflammatory or immunological processes, but mostly during autoimmune thyroiditis [18]. Our patient, being a female with HT, had risk factors for developing PTL, however, PTL is extremely rare in teenagers [19]. Ultrasonography is the first line diagnostic investigation in thyroid enlargement and nodules [11]. Based on sonographic patters thyroid lymphomas can be classified as nodular, diffuse, or mixed [7, 11]. Enhanced posterior echoes are present in all three types and distinguishes HT from other types of benign thyroid lesions [11, 20]. Both markedly hypoechoic masses or diffuse heterogeneous hypoechoic parenchyma have been found to be suspicious of PTL in different studies [7, 20, 21]. Hypoechoic echoes are also present in HT which complicates the ultrasonic differentiation between PTL and HT [20, 21]. In this case, the ultrasound revealed hypoechogenic parenchyma, which was reported as HT rather than malignancy.
Although histopathology remains the standard for the diagnosis of PTL, it is difficult to distinguish from other pathologies including lymphocytic thyroiditis and anaplastic carcinoma of the thyroid [15]. The accuracy of fine needle aspiration (FNA) has increased with the introduction of new methods including immunohistochemical staining and the detection of immunoglobuline rearrangements using PCR technique [2, 11, 15]. Differentiating between PTL and a pre-existing autoimmune thyroiditis remains difficult on FNA as the architecture cannot be assessed on cytology; additionally the high coincidence within the same gland results in higher false-negative rates due to sampling error [7, 11]. With inconclusive FNA results, a core biopsy, incisional biopsy or even a thyroidectomy is needed [2, 11, 15]. In our case, a thyroidectomy was performed, which is normally reserved when other techniques prove insufficient for diagnosis.
Hashimoto’s thyroiditis, also known as autoimmune thyroiditis or chronic lymphocytic thyroiditis, is the most common cause of autoimmune thyroid disease and acquired hypothyroidism in children and adolescents [9, 22, 23]. HT is estimated to have a prevalence in pediatrics of 1-2% with a peak frequency in adolescence [9, 24]. HT is characterized by lymphocytic infiltration of the thyroid gland [24]. This non-native lymphoid tissue can undergo malignant transformation resulting in PTL [18]. The most common complaint at diagnosis is a goitre, present in 40-70% in children and patients are usually euthyroid or hypothyroid at presentation, rarely hyperthyroid [23, 24]. HT can be accompanied with structural changes over time; benign thyroid nodules, carcinoma, and, uncommonly, primary non-Hodgkin lymphoma can develop. The relationship between HT and malignancy is poorly defined [9]. The risk for PTL in pediatric patients with HT is not known. However, adults have a 40-80-fold increased risk to develop PTL during HT [15]. As HT is a well-established risk factor for PTL in adults, we suspect the same relationship to be true for the pediatric population. HT is 2-8 times more common in girls, the female predominance continues in adulthood reflecting in an increased incidence of PTL in females [23, 24]. Studies conducted to review HT as a risk factor for development of papillary thyroid carcinoma in pediatric patients do support this relationship [9, 14, 25].
In contrast, a study performed by Radetti et al. concluded that HT increases the risk of developing thyroid nodules, but not of thyroid cancer [8]. The relationship between HT and pediatric thyroid cancer, including PTL, remains debatable, warranting further research. Surgery is not the first line treatment with benign pathology and should be reserved for selected cases [11, 15]. Our patient with HT underwent a thyroidectomy due to enlarging goitre with insufficient response to thyroid supplements. Surgical therapy for HT is usually reserved for relief of compressive symptoms or malignancy [23]. The optimal therapeutic approach for PTL remains controversial due to a lack of large prospective studies [6, 7, 11, 15]. Local control can be obtained by radiotherapy, surgery or both, while chemotherapy is added for control of disseminated disease as was the case in our patient [15]. For DLBCL the combination of chemotherapy and radiotherapy is most commonly used [7, 11, 15].
Our patient was diagnosed with β-thalassemia minor/intermedia after completion of chemotherapy. In literature, there are cases reported of β- thalassemia associated with malignancies, predominantly liver cancer and hematologic malignancies. It has not yet been established whether the simultaneous occurrence is purely coincidental, or if there is a causal relation [26, 27]. Karimi et al. concluded that the age-specific rates of cancer incidence in thalassemia patients were greater than those in the general population [28]. One of the theories suggesting a pathogenic correlation between thalassemia and cancer is iron-induced oxidative damage due to iron overload from chronic transfusion [26]. In contrast, our patient was transfusion independent, but had an iron deficiency. The occurrence of lymphoma in thalassemia has rarely been reported none of which were PTL [29, 30].
No associations have been found between thyroid disease or malignancy and Usher Syndrome, however, there are case reports linking retinitis pigmentosa to autoimmune diseases and thyroid dysfunction [31].
Conclusion
PTL is extremely rare in children and although the relation is not yet well-established, it seems likely that paediatric patients with HT are at increased risk to develop thyroid cancer. HT in children is common, warranting a better understanding of the risk of malignancy in these patients. Malignancies should always be considered in children with HT in the presence of a rapid growing goitre. In this case, our patient also had β- thalassemia and Usher syndrome, illustrating that various diseases can present in a single patient synchronously. Although rare in the presence of syndromes, malignancies should always be considered in the differential diagnoses and unexplained symptoms should be investigated.
Article Info
Article Type
Case ReportPublication history
Received: Wed 06, May 2020Accepted: Mon 18, May 2020
Published: Fri 29, May 2020
Copyright
© 2023 Yeleni Eelen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.05.11
Author Info
Jaques van Heerden Joris Verlooy Koen Norga Thomas Tousseyn Yeleni Eelen
Corresponding Author
Yeleni EelenDepartment of Paediatrics, Antwerp University Hospital, University of Antwerp, Belgium
Figures & Tables


References
- Sharma A, Jasim S, Reading CC, Ristow KM, Villasboas Bisneto JC et al. (2016) Clinical Presentation and Diagnostic Challenges of Thyroid Lymphoma: A Cohort Study. Thyroid 26: 1061-1067. [Crossref]
- Pavlidis E T, Pavlidis T E (2019) A Review of Primary Thyroid Lymphoma: Molecular Factors, Diagnosis and Management. J Invest Sur 32: 137-142. [Crossref]
- Harach H R, Williams E D (1995) Childhood thyroid cancer in England and Wales. Br J Cancer 72: 777-783. [Crossref]
- Gosepath J, Spix C, Talebloo B, Blettner M, Mann WJ (2007) Incidence of childhood cancer of the head and neck in Germany. Ann Oncol 18: 1716-1721. [Crossref]
- Cooper K, Gangadharan, A, Arora R S, Shukla R, Pizer B (2014) Burkitt lymphoma of thyroid gland in an adolescent. Case Rep Pediatr 2014: 187467. [Crossref]
- Walsh S, Lowery A J Evoy, D McDermott E W, Prichard R S (2013) Thyroid Lymphoma: Recent Advances in Diagnosis and Optimal Management Strategies. Oncologist 18: 994-1003. [Crossref]
- Mancuso S, Carlisi M, Napolitano M, Siragusa S (2018) Lymphomas and thyroid: Bridging the gap. Hematol Oncol . [Crossref]
- Radetti G, Loche S, D'Antonio V, Salerno M, Guzzetti C et al. (2019) Influence of Hashimoto Thyroiditis on the Development of Thyroid Nodules and Cancer in Children and Adolescents. J Endocr Soc 3: 607-616. [Crossref]
- Penta L, Cofini M, Lanciotti L, Leonardi A, Principi N et al. (2018) Hashimoto’s disease and thyroid cancer in children: Are they associated? Front Endocrinol (Lausanne) 9: 565. [Crossref]
- Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M et al. (2008) Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med 162: 526-531. [Crossref]
- Stein S A & Wartofsky L (2013) Primary thyroid lymphoma: A clinical review. J Clin Endocrinol Metabol 98: 3131-3138. [Crossref]
- Agrawal R & Wang J (2009) Pediatric follicular lymphoma: A rare clinicopathologic entity. Arch Pathol Lab Med 133: 142-146. [Crossref]
- Louissaint A, Schafernak KT, Geyer JT, Kovach AE, Ghandi M et al. (2016) Pediatric-type nodal follicular lymphoma: A biologically distinct lymphoma with frequent MAPK pathway mutations. Blood 128: 1093-1100. [Crossref]
- Won J H, Lee J Y, Hong H S & Jeong S H (2018) Thyroid nodules and cancer in children and adolescents affected by Hashimoto’s thyroiditis. Br J Radiol 91 :20180014 [Crossref]
- Peixoto R, Correia Pinto J, Soares V, Koch P & Taveira Gomes (2017) A Primary thyroid lymphoma: A case report and review of the literature. Ann Med Surg (Lond) 13: 29-33. [Crossref]
- Alzouebi M, Goepel JR, Horsman J M, Hancock B W (2012) Primary thyroid lymphoma: The 40-year experience of a UK lymphoma treatment centre. Int J Oncol 40: 2075-2080. [Crossref]
- Verma D, Puri V, Agarwal S & Bhaskar A (2014) Primary thyroid lymphoma: A rare disease. J Cytol 31: 218-220. [Crossref]
- Allaoui M, Benchafai I, Mahtat el M, Regragui S, Boudhas A et al. (2016) Primary Burkitt lymphoma of the thyroid gland: case report of an exceptional type of thyroid neoplasm and review of the literature. BMC Clin Pathol 16: 6. [Crossref]
- Travaglino A, Pace M, Varricchio S, Insabato L, Giordano C et al. (2020) Hashimoto Thyroiditis in Primary Thyroid Non-Hodgkin Lymphoma. Am J Clin Pathol 153: 156-164. [Crossref]
- Nam M, Shin JH, Han BK, Ko EY, Ko ES et al. (2012) Thyroid lymphoma: correlation of radiologic and pathologic features. J Ultrasound Med 31: 589-594. [Crossref]
- Ma B, Jia Y, Wang Q & Li X (2014) Ultrasound of primary thyroid non-Hodgkin’s lymphoma. Clin Imaging 38: 621-626. [Crossref]
- Ramesh B G, Bhargav PR, Rajesh BG, Vimala Devi N, Vijayaraghavan R et al. (2015) Genomics and phenomics of Hashimoto’s thyroiditis in children and adolescents: a prospective study from Southern India. Ann Transl Med 3: 280. [Crossref]
- Sar E, Karaoglu A & Yesilkay E (2011) Hashimoto’s Thyroiditis in Children and Adolescents. in Autoimmune Disorders - Current Concepts and Advances from Bedside to Mechanistic Insights (InTech). doi:10.5772/24755.
- Özsu E, Mutlu R G Y, Çizmeci F & Hatun Ş (2011) Hashimoto tiroiditli hastalari{dotless}mi{dotless}zi{dotless}n özellikleri. Turk Pediatri Arsivi 46: 252-255.
- Zeng R, Zhao M, Niu H, Yang KX, Shou T et al. (2018) Relationship between Hashimoto’s thyroiditis and papillary thyroid carcinoma in children and adolescents. Eur Rev Med Pharmaco Sci 22: 7778-7787. [Crossref]
- Benetatos L, Alymara V, Vassou A & Bourantas K L (2008) Malignancies in beta-thalassemia patients: a single-center experience and a concise review of the literature. Int J Lab Hematol 30: 167-172. [Crossref]
- Otrock Z K, Shamseddine A I & Taher A T (2006) Non-Hodgkin disease in β-thalassemia major. Am J Hematol 81: 62-64. [Crossref]
- Karimi M, Giti R, Haghpanah S, Azarkeivan A, Hoofar H et al. (2009) Malignancies in patients with β-thalassemia major and β-thalassemia intermedia: A multicenter study in Iran. Pediatr Blood Cancer 53: 1064-1067. [Crossref]
- Patir P, Soyer N & Saydam G (2016) Beta-thalassemia Major and Non-Hodgkin Lymphoma. Hemoglobin 26: 219-225.
- Alavi S, Safari A, Sadeghi E & Amiri S (2013) Hematological malignancies complicating β-thalassemia syndromes: A single center experience. Blood Res 48: 149-151. [Crossref]
- Nye F J & Evans W H (1977) Retinitis pigmentosa and autoimmune endocrine abnormalities in identical twins. Br Med J 1: 616-617. [Crossref]
