Predisposing Factors to HBV Among Pregnant Women Attending Some Hospitals in Suburbs of Kano, Nigeria
A B S T R A C T
Hepatitis B virus (HBV) when transmitted vertically can be severe on neonates and life threatening. Among others, risk factors for HBV include unprotected sex, needle-stick injuries and blood transfusion. The study was conducted to determine the seroprevalence of HBV markers and associated risk factors among one hundred and sixty consenting pregnant women attending some hospitals in Kano, Nigeria. Using enzyme-linked immunoassay, sera were screened for HBV sero-markers and structured questionnaires were administered to obtain sociodemographic data and possible predisposing factors to HBV infection. Of the five HBV markers known, participants tested positive for four, which include HBsAg, HBsAb, HBeAb and HBcAb. All were seronegative for HBeAg. Ninety three percent (93.1%) tested positive for at least one HBV marker and 6.9% were seronegative for all markers. Among those that tested positive for HBsAg, 54.5% (p=0.33) were housewives, 36.4% (p=0.53) had only primary school education, 72.7% (p=0.14) were middle-class, none had previous knowledge of HBV infection and its mode of transmission, 54.5% (p=0.14) regularly shares sharp objects, 45.5% (p=0.37) had ear or nose piercing, and 9.1% (p=0.01) regularly shares towel and underwear. A large percentage of the study group had history of the infection while only 1.3% of the subjects were vaccinated. Sociodemographic background of the participants, low vaccination coverage and certain risk factors like the sharing of unsterilized sharp objects seem to aid the moderately high prevalence of HBV in this study. The study also revealed that the risk of mother-to-child HBV transmission is low in the study area and that incomplete vaccination may not confer artificial immunity against HBV infection.
Keywords
HBV, predisposing factors, serological markers , seroprevalence, pregnancy
Introduction
It is estimated that about 2 billion people have been exposed to Hepatitis B virus (HBV) at some time in their lives and approximately 39 million people have died because of HBV related infections since it was defined in 1981 [1, 2]. Also, more than 686, 000 people continue to die annually due to complications of HBV infection while about 257 million are living with HBV infection. Globally, the prevalence of HBsAg is 3.61% and it is estimated that 170 million people that have chronic hepatitis B (CHB) reside in Africa [3-6]. The infection is endemic in Nigeria with an estimated 3-12% of her total population being chronic-carriers and about 15 million being seropositive for HBsAg. Likewise, 7% of pregnant women in Nigeria have HBV infection [7-9]. Without immunization, neonates born to HBV chronically infected mothers have about 70-90% risk of the infection progressing to a chronic phase and thus developing chronic liver disease. In contrast, only 20% to 30% of children exposed between ages 1 and 5 years, and fewer than 5% of adults, become infected [7, 10, 11]. Usually certain risk factors like the transfusion of HBV infected blood or blood products, intravenous drug abuse, needle-stick and sharp object injury, ear-piercing, tattooing and through close interpersonal contact with an infected household member helps in transmitting HBV. Infection may also spread by fomites, sharing of toothbrushes and sexual contact with infected persons [9, 12]. The aim of the study is to determine the associated risk factors of HBV among pregnant women attending some hospitals in the suburbs of Kano State, Nigeria in relation to their HBV status.
Materials & Methods
I Study Area and Population
The study area consisted of Gaya, Sumaila and Wudil Local Government Areas, Kano State, Nigeria. According to the National Population Commission, Kano has an estimated population of 9, 401, 288 and its coordinates is 11.7574 oN, 8.6601 oE [13]. The cohort was made up of 160 pregnant women attending antenatal clinics of the three General hospitals in these suburbs.
II Study Design and Sample Size Determination
The research was a cross-sectional study involving the use of a structured questionnaire which was administered to each participant in order to obtain information on their sociodemographic and other relevant data considered as risk factors. The minimum and estimated sample size were determined using n=Z2P (1 - P)/d2 and Ns=n/arr respectively at 95% confidence interval with an anticipated response rate of 90% [14, 15].
III Medical Ethics and Inclusion Criteria
In accordance with the Helsinki code of ethics for biomedical research involving human subjects, ethical approval was obtained from the office of the Honorable Commissioner for Health, Kano, through its Ethics Committee. Only registered patients and those whose consent were sought were involved in the study.
IV Sample Collection, Processing and Analysis of Blood Sample
Blood samples were collected, processed and analyzed serologically using rapid test and enzyme linked immunosorbent assay as described by Abulude et al. [16].
V Data Analysis
Statistical tests were performed on the data obtained from test results and questionnaire using IBM SPSS Version 20. Pearson�s Chi-square test was used to evaluate the difference in proportion between HBV serologic markers and sociodemographic/risk factors and p-value of equal or less than 0.05 at 95% confidence interval (CI) was considered as statistically significant.
Results
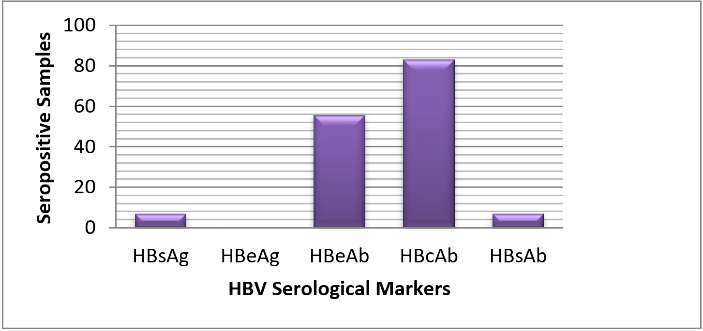
Out of 182 participants that were selected, only 160 took part in the study by returning valid questionnaires and donating blood samples. The remaining 22 withdrew their consent for their own personal reasons. Of the five known HBV serological markers, only four were encountered in this study. Large percentage of the participants (93.1%) were seropositive for at least one HBV marker while the remaining 6.9% were seronegative. As shown in (Figure 1), out of the 160, 11 (6.9%) tested positive for HBsAg, 11 (6.9%) tested positive for HBsAb, 89 (55.6%) tested positive for HBeAb, 132 (83.0%) tested positive for HBcAb in various combinations and none tested positive for HBeAg.
Figure 1: Distribution of HBV serological markers among the cohort in the study area.
The relationship between HBV markers and sociodemographic information of the participants were present in (Table 1). Out of 6.9% subjects that tested positive to HBsAg, the highest frequency of HBsAg was observed within the age range 20-29 years (54.5%; p=0.90), primary education (36.4%; p=0.53), middle class (72.7%; p=0.14) and housewives (54.5%; p=0.33). The distributions of other HBV markers among these sociodemographic variables were almost similar to those of HBsAg.
Table 1: Distribution of HBV seromarkers among pregnant women with difference sociodemographic background in the study area.
|
Socio Demographic Factors |
Freq (%) |
Sero+ve= 11 HBsAg (%) |
X2 (p-value) |
Sero+ve= 11 HBsAb (%) |
X2(pvalue) |
Sero+ve=0 HBeAg (%) |
Sero+ve= 89 HBeAb (%) |
X2 (pvalue) |
Sero+ve= 133 HBcAb (%) |
X2 (pvalue) |
|
Age Range |
||||||||||
|
20-19 |
45 (28.1) |
3 (27.3) |
2 (18.2) |
0 (0.0) |
26 (29.2) |
41 (30.8) |
||||
|
20-29 |
86 (53.8) |
6 (54.5) |
9 (81.8) |
0 (0.0) |
50 (56.2) |
65 (48.9) |
||||
|
30-39 |
23 (14.4) |
2 (18.2) |
0.56a (0.90) |
0 (0.0) |
4.28a (0.23) |
0 (0.0) |
11 (12.4) |
2.08a (0.56) |
21 (15.8) |
3.39a (0.33) |
|
40-49 |
6 (3.8) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
2 (2.2) |
6 (4.5) |
||||
|
50-59 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Education |
||||||||||
|
No Formal Education |
49 (30.6) |
2 (18.2) |
2 (18.2) |
0 (0.0) |
22 (24.7) |
46 (34.6) |
||||
|
Primary |
29 (18.1) |
4 (36.4) |
1 (9.1) |
0 (0.0) |
16 (18.0) |
23 (17.3) |
||||
|
Secondary |
26 (16.3) |
2 (18.2) |
3.13a (0.53) |
2 (18.2) |
3.01a (0.55) |
0 (0.0) |
14 (15.7) |
6.17a (0.18) |
17 (12.8) |
11.16a (0.02)* |
|
Tertiary |
3 (1.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Quranic only |
53 (33.1) |
3 (27.3) |
6 (54.5) |
0 (0.0) |
37 (41.6) |
47 (35.3) |
||||
|
Socio-Economic Status |
||||||||||
|
Poor |
78 (48.8) |
3 (27.3) |
2 (18.2) |
0 (0.0) |
36 (40.4) |
70 (52.6) |
||||
|
Middle class |
82 (51.3) |
8 (72.7) |
2.18a (0.14) |
9 (81.8) |
4.41a (0.03)* |
0 (0.0) |
53 (59.6) |
5.53a (0.01)* |
63 (42.4) |
3.74a (0.05)* |
|
Rich |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Family Type |
||||||||||
|
Monogamous |
107 (66.9) |
8 (72.7) |
0.18a (0.66) |
7 (63.6) |
0.05a (0.81) |
0 (0.0) |
61 (68.5) |
0.25a (0.61) |
85 (63.9) |
5.43a (0.02)* |
|
Polygamous |
53 (33.1) |
3 (27.3) |
4 (36.4) |
0 (0.0) |
28 (31.5) |
48 (36.1) |
||||
|
Marital Status |
||||||||||
|
Single |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Married |
160 (100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Divorced/ Seperated |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Widow |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Occupation |
||||||||||
|
Housewife |
121 (75.6) |
6 (54.5) |
7 (63.6) |
0 (0.0) |
61 (68.5) |
102 (76.7) |
||||
|
Trader |
37 (23.1) |
5 (45.5) |
4 (36.4) |
0 (0.0) |
27 (30.3) |
30 (22.6) |
||||
|
Professional |
1 (6.0) |
0 (0.0) |
3.38a (0.33) |
0 (0.0) |
1.26a (0.73) |
0 (0.0) |
0 (0.0) |
9.86a (0.02)* |
0 (0.0) |
4.97a (0.17) |
|
Farmer |
1 (6.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Others |
0 (0.0) |
0 (0.0) |
|
0 (0.0) |
|
0 (0.0) |
1 (1.2) |
|
1 (0.8) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
Note: Poor=living on ≤US$1.25/day, Middle class=living on ≤US$2-13US$/day, Rich= living on ˃US$13/day [20].
Table 2: Distribution of HBV markers among the cohort in relation to their knowledge of HBV infection & transmission, vaccination, multiple marriages, living in overcrowded conditions, history of rape, blood transfusion and STDs.
|
Risk Factors |
Freq (%) |
Sero+ve=11 HBsAg (%) |
X2 (p-value) |
Sero+ve=11 HBsAb (%) |
X2 (p-value) |
Sero+ve=0 HBeAg (%) |
Sero+ve=89 HBeAb (%) |
X2 (p-value) |
Sero+ve=133 HBcAb (%) |
X2 (p-value) |
|
Previous Knowledge of HBV Infection |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
0.15a (0.69) |
1 (9.1) |
5.88a (0.01)* |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
0 (0.0) |
9.54a (0.05)* |
|
No |
158 (98.7) |
11 (100.0) |
10 (90.9) |
0 (0.0) |
89 (100.0) |
133 (100) |
||||
|
Knowledge of HBV Transmission |
||||||||||
|
Yes |
0(0.0) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|||
|
No |
160(100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Vaccination |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
0.26a (0.63) |
1 (9.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|||
|
No |
158 (98.7) |
11 (100) |
10 (90.9) |
0 (0.0) |
89 (100.0) |
133 (100) |
||||
|
Rape |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
3.34a (0.06) |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
1 (0.8) |
1.48a (0.22) |
|
No |
158 (98.7) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
132 (99.2) |
||||
|
Multiple Marriages |
||||||||||
|
Yes |
1 (0.6) |
0 (0.0) |
0.07a (0.78) |
0 (0.0) |
0.07a (0.78) |
0 (0.0) |
1 (1.1) |
0.80a (0.37) |
1 (0.7) |
0.21a (0.96) |
|
No |
159 (99.4) |
11 (100) |
11 (100) |
0 (0.0) |
88 (98.8) |
132 (99.2) |
||||
|
Living in overcrowded conditions |
||||||||||
|
Yes |
30 (18.7) |
1 (9.1) |
0.72a (0.39) |
4 (36.3) |
2.40a (0.12) |
0 (0.0) |
19 (21.3) |
0.88a (0.34) |
24 (18.1) |
0.44a (0.50) |
|
No |
130 (81.3) |
10 (90.9) |
7 (63.6) |
0 (0.0) |
70 (78.6) |
109 (81.9) |
||||
|
Blood Transfusion |
||||||||||
|
Yes |
17 (10.6) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
0 (0.0.) |
3.34a (0.06) |
0 (0.0) |
0.43a (0.51) |
|
No |
143 (89.4) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
89 (100.0) |
133 (100.0) |
||||
|
History of STI/STD |
||||||||||
|
Yes |
3 (1.9) |
1 (9.1) |
3.34a (0.06) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
2 (1.5) |
0.53a (0.46) |
|
No |
157 (98.1) |
10 (90.9) |
|
11 (100) |
|
0 (0.0) |
88 (98.8) |
|
131 (98.8) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
Table 3: Distribution of HBV markers in relation to sharing of unsterilized personal items, scarification, surgery and dental procedure among the cohort.
|
Risk Factors |
Freq (%) |
Sero+ve=11 HBsAg (%) |
X2 (pvalue) |
Sero+ve=11 HBsAb (%) |
X2 (pvalue) |
Sero+ve=0 HBeAg (%) |
Sero+ve= 89 HBeAb (%) |
X2 (pvalue) |
Sero+ve=133 HBcAb (%) |
X2 (pvalue) |
|
Sharing of Unsterilized Sharp Objects |
||||||||||
|
Yes |
68 (42.5) |
6 (54.5) |
2.15a (0.14) |
1 (9.1) |
5.39a (0.02)* |
0 (0.0) |
32 (35.9) |
3.51a (0.06) |
56 (42.1) |
0.002a (0.96) |
|
No |
92 (57.5) |
5 (45.4) |
10 (90.9) |
0 (0.0) |
57 (64.0) |
77 (57.8) |
||||
|
Sharing of Toothbrush/Chewing Stick |
||||||||||
|
Yes |
10 (6.3) |
2 (18.1) |
2.87a (0.09) |
1 (9.1) |
0.16a (0.68) |
0 (0.0) |
3 (3.3) |
0.13a (0.71) |
5 (3.7) |
3.74a (0.05)* |
|
No |
150 (93.7) |
9 (81.8) |
10 (90.9) |
0 (0.0) |
86 (96.6) |
128 (96.2) |
||||
|
Sharing of Towel/ Underwear |
||||||||||
|
Yes |
2 (1.3) |
1 (9.1) |
5.88a (0.01)* |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
1 (0.8) |
1.48a (0.22) |
|
No |
158 (93.7) |
10 (90.9) |
11 (100) |
0 (0.0) |
89 (100) |
132 (99.2) |
||||
|
Intravenous Drug User |
||||||||||
|
Yes |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
No |
160(100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Tatoo/ Incision |
||||||||||
|
Yes |
3 (1.9) |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
3 (2.3) |
0.64a (0.42) |
|
No |
157 (98.1) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
88 (98.9) |
130 (97.7) |
||||
|
Dental Procedure |
||||||||||
|
Yes |
4 (2.5) |
0 (0.0) |
0.77a (0.37) |
0 (0.0) |
0.30a (0.58) |
0 (0.0) |
0 (0.0) |
1.55a (0.21) |
1 (0.8) |
0.16a (0.68) |
|
No |
156(98.7) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
89 (100.0) |
132 (99.2) |
||||
|
Surgery |
||||||||||
|
Yes |
3 (1.9) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
3 (2.3) |
0.64a (0.42) |
|
No |
157 (98.1) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
88 (98.9) |
130 (97.7) |
||||
|
Tribal Mark |
||||||||||
|
Yes |
93 (58.1) |
2 (18.2) |
0.30a (0.58) |
4 (36.4) |
2.29a (0.13) |
0 (0.0) |
45 (50.6) |
3.41a (0.06) |
79 (59.4) |
3.25a (0.07) |
|
No |
67 (41.9) |
9 (81.8) |
7 (63.6) |
0 (0.0) |
44 (49.4) |
54 (40.6) |
||||
|
Ear/Nose Piercing |
||||||||||
|
Yes |
67 (41.9) |
5 (45.4) |
0.77a (0.37) |
6 (54.5) |
4.61a (0.03)* |
0 (0.0) |
41 (46.1) |
9.85a (0.05)* |
51 (38.3) |
0.09a (0.76) |
|
No |
93 (58.1) |
6 (54.5) |
|
5 (45.4) |
|
0 (0.0) |
48 (53.9) |
|
82 (61.6) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
As shown in (Table 2), none of the participants with HBsAg had previous knowledge of HBV infection and its transmission (p=0.69). Also (Table 3) showed that among those with HBsAg, 18.2% (p=0.09) shares toothbrush with others, 54.5% (p=0.14) shares unsterilized sharp objects, and 45.4% (p=0.37) pierced either ear or nose.
Discussion
In this study, the level of awareness of HBV infection among the study population was very poor, as almost all the participants claimed ignorance of the disease and its mode of transmission. In a study conducted in Abakaliki to test the knowledge and awareness of HBV infection among pregnant women, 24.8% of the study group knew that HBV is a viral infection affecting the liver, 6.8% thought it is an eye disease, while 52.5% do not know what it is [9]. The lack of knowledge of HBV infection and its mode of transmission among the participants in this study may be due to the suburban localities in which the research was conducted which make access to public health information scanty.
The occurrence of HBsAg varies with sociodemographic factors of the study group, as well as certain risk factors. The highest prevalence of HBsAg recorded among participants within the age range 20-29 conforms to the findings of Ndams et al., where out of the HBsAg seropositives, the majority falls within the age brackets of 21-30 years but contrasts the findings of Mbaawuaga et al., with the highest prevalence seen in age range 11-19 [17, 18]. The reason for this may be due to the fact that this is the age bracket at which most women are likely to get married and start having children, thus becoming sexually and socially active. It was observed that all the subjects in this study were married. This high percentage of married women is similar to the findings of Bayo et al., where 96.2% of the subjects were also married [19]. This is expected of a typical Nigerian society where pregnancy outside marriage is generally not acceptable. This study also revealed that 72.7% and 23.3% of those that tested positive for HBsAg were in a monogamous and polygamous relationship respectively. Family type seems to play a role in HBV transmission in polygamous homes where the infection can be spread easily among spouses.
In this study, HBsAg was more prevalent among the middle-class than the lower-class. This contrasts the findings of Ugwuja and Ugwu, where more infections were found in participants from lower-class than in middle or upper- socioeconomic classes [12]. Based on the purchasing power parity (PPP), Chen and Ravallion defined the poor in developing countries as those living below the US$1.25 poverty line, while the middle class is defined as living in a household with per capita consumption between US$2 and US$13 per day at PPP [20]. The higher prevalence among these social classes considered in the study may be due to the fact that socioeconomic background of the inhabitants of the study area somehow affects their access to the basic health information and knowledge generally as poverty tend to influence people's attitude, lifestyle their condition of living. Interestingly, the highest prevalence of HBsAg was found among those that were housewives. This contrasts the findings of Ndako et al., where the percentage of housewives with HBV infection is very few (6.7%). Olaitan and Zamani, in their study found out that business women had a higher prevalence of HBsAg than other women studied [21, 23]. This high prevalence seen in this study may be due to lack of exposure as housewives are usually confined indoor most times, thereby depriving them of access to the basic information and knowledge of HBV infection and its mode of transmission. The prevalence of HBsAg observed among those that have no formal education in this study is low compared with those with only primary or Quranic education. This finding is almost similar to that of Ndams et al., where the illiterate women constituted only15.9% but contrast the finding of studies where HBsAg prevalence was higher among the illiterates [17, 22-24]. Lack of formal education or having just a modicum of it seems to limit access to basic information especially pertaining to health. In this study, none of the sociodemographic factors showed significant association with HBsAg seropositivity. With regard to risk factors, a very high prevalence of HBsAg seropositivity was seen among the participants that regularly shares unsterilized sharp objects. Though not statistically significant, it has the highest percentage among the risk factors in this study. The implication is that this is probably one of the most possible routes of transmitting HBV infection among the study group. Similarly, Ndako et al. in their study reported that the sharing of unsterilized sharp instruments top the risk factors while Ugwuja and Ugwu study also revealed that the major route of HBV transmission in their study group was unsafe injection [12, 21]. This practice may be due to the communal lifestyle of the study group and lack of knowledge about the danger of sharing unsterilized sharp objects even among family members. A large percentage of the study group had either ear or nose piercing or both. Since no further information was provided on how and when such piercing was done, it is difficult to make a reasonable conclusion on this, however, in rural area most piercing is usually performed domestically and as such there is likelihood that unsterilized objects might have been used for the piercing. Likewise, few of those with HBsAg seropositivity had tribal marks. Owing to the relatively small percentage, this is unlikely to be a mode of HBV transmission except when the same unsterilized object used for the scarification is used on many people. This finding is similar to that of Ugwuja and Ugwu, where it was reported that 13.3% of those that tested positive for HBsAg had tribal marks but contrasts the study of Opaleye et al. that shows that among those with tribal marks, none were positive for HBsAg [12, 25]. Also, few participants claimed that they regularly shares toothbrush or chewing-stick. Since most people often experience bleeding gums that arise from teeth brushing, sharing of a toothbrush could thus help in transmitting HBV infection from one person to another. None of the subjects that tested positive for HBsAg had a history of blood transfusion. This finding contrast that of Pennap et al., where the prevalence of the HBsAg was higher among those that had a history of blood transfusion (20.8%) and that of Oladeinde et al., with the prevalence of 2.2%. Likewise, none of the subjects that tested positive for HBsAg had a history of surgery [26, 27]. In a similar study conducted by Olokoba et al. in Yola, the majority of the women sampled never had a blood transfusion (93.5%) and never had surgery (84.4%). Ugwuja and Ugwu study revealed in Iran, which showed that 6.7% of their subjects had a history of blood or blood products transfusions [12, 28]. That means that blood transfusions and surgery were two of the few means of contracting HBV in Kano State, Nigeria. Also, none of those that tested positive for HBsAg had a history of rape, intravenous drug use, dental procedure and multiple marriages with no significant association with HBsAg seropositivity. Also, none of the participants wear tattoos on their body. This finding is similar to that of Afzali et al., study in Iran, which showed that there was no history of dental procedure or skin tattoo among the HBsAg positive cases [22]. In this study, among those that are positive for HBsAg, few regularly share towels and/or underwear, lived in a crowded condition, and had a history of sexually transmitted infections (STIs). Poor living condition is known to contribute to the spread of communicable diseases. Likewise, sharing of certain personal items like towel can promote the easy transmission of the HBV infection, especially when there is a wound or cut on the skin of an infected person. Like HBsAg, other HBV sero-markers also prevailed more among the age range 20-29 years and housewives while the risk factors varied considerably among them.
The findings from this study revealed that only two participants received HBV vaccine. Out of these two, one was tested positive for HBsAb only. This particular participant was successfully vaccinated with the required three dosages of vaccine, while the other participant received only a dose of the vaccine and thus tested negative for all the five markers. This may be due to the inability of a single dosage to induce an appropriate immune response. This corroborates the findings of Opaleye et al. in which only 8 (53.3%) out of the 15 pregnant women with the history of hepatitis B vaccination were positive for HBsAb [25]. Seropositivity only to HBsAb indicates immunity due to HBV vaccination. That showed that only 0.6% of the study population had been successfully vaccinated. Also, HBsAb arises late during infection, usually during recovery or convalescence after clearance of HBsAg. HBsAb persist after recovery, being the antibody associated with immunity against HBV. About 10% and 15% of patients who recover from HBV infection do not develop detectable HBsAb alone as a marker of previous infection with HBV, whereas HBcAb testing is used to assess immunity and response to HBV vaccine. Seropositivity to HBsAb means the person is no more infectious. The presence of HBsAb prevents re-infection and transmission because it is able to neutralize viral infectivity [29].
Conclusion
The relatively high occurrence of HBV among this study population calls for an intervention strategy and the need for all stakeholders to brace-up to the challenge of embarking on vaccination against the HBV infection in Nigeria. Pregnant women should undergo routine screening for HBsAg, and babies born to women who tested positive for HBsAg should be immunized with hepatitis B vaccine. Also, hepatitis B immunoglobulin should be administered to the baby if the mother is positive for HBeAg. Furthermore, there is a need for improvements in certain lifestyle patterns such as sharing of towel, underwear, unsterilized sharp objects, toothbrush, and so on, with a view to prevent transmission of the HBV infection.
Conflicts of Interest
There are no conflicts of interest.
Financial Support
None.
Acknowledgements
We would like to thank the staff of the hospitals used for this research for the necessary technical assistance rendered. Also, we like to show our gratitude to all those that participated in this study.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 23, Sep 2019Accepted: Wed 09, Oct 2019
Published: Wed 20, Nov 2019
Copyright
© 2023 Olatunji Ayodeji Abulude. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2019.01.02
Author Info
Farouk Umar Sadisu Ismai’la Ahmed Olatunji Ayodeji Abulude
Corresponding Author
Olatunji Ayodeji AbuludeDepartment of Biological Sciences, Faculty of Science, Nigeria Police Academy, Wudil, P. M. B. 3474 Kano State, Nigeria
Figures & Tables
Table 1: Distribution of HBV seromarkers among pregnant women with difference sociodemographic background in the study area.
|
Socio Demographic Factors |
Freq (%) |
Sero+ve= 11 HBsAg (%) |
X2 (p-value) |
Sero+ve= 11 HBsAb (%) |
X2(pvalue) |
Sero+ve=0 HBeAg (%) |
Sero+ve= 89 HBeAb (%) |
X2 (pvalue) |
Sero+ve= 133 HBcAb (%) |
X2 (pvalue) |
|
Age Range |
||||||||||
|
20-19 |
45 (28.1) |
3 (27.3) |
2 (18.2) |
0 (0.0) |
26 (29.2) |
41 (30.8) |
||||
|
20-29 |
86 (53.8) |
6 (54.5) |
9 (81.8) |
0 (0.0) |
50 (56.2) |
65 (48.9) |
||||
|
30-39 |
23 (14.4) |
2 (18.2) |
0.56a (0.90) |
0 (0.0) |
4.28a (0.23) |
0 (0.0) |
11 (12.4) |
2.08a (0.56) |
21 (15.8) |
3.39a (0.33) |
|
40-49 |
6 (3.8) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
2 (2.2) |
6 (4.5) |
||||
|
50-59 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Education |
||||||||||
|
No Formal Education |
49 (30.6) |
2 (18.2) |
2 (18.2) |
0 (0.0) |
22 (24.7) |
46 (34.6) |
||||
|
Primary |
29 (18.1) |
4 (36.4) |
1 (9.1) |
0 (0.0) |
16 (18.0) |
23 (17.3) |
||||
|
Secondary |
26 (16.3) |
2 (18.2) |
3.13a (0.53) |
2 (18.2) |
3.01a (0.55) |
0 (0.0) |
14 (15.7) |
6.17a (0.18) |
17 (12.8) |
11.16a (0.02)* |
|
Tertiary |
3 (1.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Quranic only |
53 (33.1) |
3 (27.3) |
6 (54.5) |
0 (0.0) |
37 (41.6) |
47 (35.3) |
||||
|
Socio-Economic Status |
||||||||||
|
Poor |
78 (48.8) |
3 (27.3) |
2 (18.2) |
0 (0.0) |
36 (40.4) |
70 (52.6) |
||||
|
Middle class |
82 (51.3) |
8 (72.7) |
2.18a (0.14) |
9 (81.8) |
4.41a (0.03)* |
0 (0.0) |
53 (59.6) |
5.53a (0.01)* |
63 (42.4) |
3.74a (0.05)* |
|
Rich |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Family Type |
||||||||||
|
Monogamous |
107 (66.9) |
8 (72.7) |
0.18a (0.66) |
7 (63.6) |
0.05a (0.81) |
0 (0.0) |
61 (68.5) |
0.25a (0.61) |
85 (63.9) |
5.43a (0.02)* |
|
Polygamous |
53 (33.1) |
3 (27.3) |
4 (36.4) |
0 (0.0) |
28 (31.5) |
48 (36.1) |
||||
|
Marital Status |
||||||||||
|
Single |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Married |
160 (100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Divorced/ Seperated |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Widow |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Occupation |
||||||||||
|
Housewife |
121 (75.6) |
6 (54.5) |
7 (63.6) |
0 (0.0) |
61 (68.5) |
102 (76.7) |
||||
|
Trader |
37 (23.1) |
5 (45.5) |
4 (36.4) |
0 (0.0) |
27 (30.3) |
30 (22.6) |
||||
|
Professional |
1 (6.0) |
0 (0.0) |
3.38a (0.33) |
0 (0.0) |
1.26a (0.73) |
0 (0.0) |
0 (0.0) |
9.86a (0.02)* |
0 (0.0) |
4.97a (0.17) |
|
Farmer |
1 (6.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
Others |
0 (0.0) |
0 (0.0) |
|
0 (0.0) |
|
0 (0.0) |
1 (1.2) |
|
1 (0.8) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
Note: Poor=living on ≤US$1.25/day, Middle class=living on ≤US$2-13US$/day, Rich= living on ˃US$13/day [20].
Table 2: Distribution of HBV markers among the cohort in relation to their knowledge of HBV infection & transmission, vaccination, multiple marriages, living in overcrowded conditions, history of rape, blood transfusion and STDs.
|
Risk Factors |
Freq (%) |
Sero+ve=11 HBsAg (%) |
X2 (p-value) |
Sero+ve=11 HBsAb (%) |
X2 (p-value) |
Sero+ve=0 HBeAg (%) |
Sero+ve=89 HBeAb (%) |
X2 (p-value) |
Sero+ve=133 HBcAb (%) |
X2 (p-value) |
|
Previous Knowledge of HBV Infection |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
0.15a (0.69) |
1 (9.1) |
5.88a (0.01)* |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
0 (0.0) |
9.54a (0.05)* |
|
No |
158 (98.7) |
11 (100.0) |
10 (90.9) |
0 (0.0) |
89 (100.0) |
133 (100) |
||||
|
Knowledge of HBV Transmission |
||||||||||
|
Yes |
0(0.0) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|||
|
No |
160(100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Vaccination |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
0.26a (0.63) |
1 (9.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|||
|
No |
158 (98.7) |
11 (100) |
10 (90.9) |
0 (0.0) |
89 (100.0) |
133 (100) |
||||
|
Rape |
||||||||||
|
Yes |
2 (1.3) |
0 (0.0) |
3.34a (0.06) |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
1 (0.8) |
1.48a (0.22) |
|
No |
158 (98.7) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
132 (99.2) |
||||
|
Multiple Marriages |
||||||||||
|
Yes |
1 (0.6) |
0 (0.0) |
0.07a (0.78) |
0 (0.0) |
0.07a (0.78) |
0 (0.0) |
1 (1.1) |
0.80a (0.37) |
1 (0.7) |
0.21a (0.96) |
|
No |
159 (99.4) |
11 (100) |
11 (100) |
0 (0.0) |
88 (98.8) |
132 (99.2) |
||||
|
Living in overcrowded conditions |
||||||||||
|
Yes |
30 (18.7) |
1 (9.1) |
0.72a (0.39) |
4 (36.3) |
2.40a (0.12) |
0 (0.0) |
19 (21.3) |
0.88a (0.34) |
24 (18.1) |
0.44a (0.50) |
|
No |
130 (81.3) |
10 (90.9) |
7 (63.6) |
0 (0.0) |
70 (78.6) |
109 (81.9) |
||||
|
Blood Transfusion |
||||||||||
|
Yes |
17 (10.6) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
1.40a (0.23) |
0 (0.0) |
0 (0.0.) |
3.34a (0.06) |
0 (0.0) |
0.43a (0.51) |
|
No |
143 (89.4) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
89 (100.0) |
133 (100.0) |
||||
|
History of STI/STD |
||||||||||
|
Yes |
3 (1.9) |
1 (9.1) |
3.34a (0.06) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
2 (1.5) |
0.53a (0.46) |
|
No |
157 (98.1) |
10 (90.9) |
|
11 (100) |
|
0 (0.0) |
88 (98.8) |
|
131 (98.8) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
Table 3: Distribution of HBV markers in relation to sharing of unsterilized personal items, scarification, surgery and dental procedure among the cohort.
|
Risk Factors |
Freq (%) |
Sero+ve=11 HBsAg (%) |
X2 (pvalue) |
Sero+ve=11 HBsAb (%) |
X2 (pvalue) |
Sero+ve=0 HBeAg (%) |
Sero+ve= 89 HBeAb (%) |
X2 (pvalue) |
Sero+ve=133 HBcAb (%) |
X2 (pvalue) |
|
Sharing of Unsterilized Sharp Objects |
||||||||||
|
Yes |
68 (42.5) |
6 (54.5) |
2.15a (0.14) |
1 (9.1) |
5.39a (0.02)* |
0 (0.0) |
32 (35.9) |
3.51a (0.06) |
56 (42.1) |
0.002a (0.96) |
|
No |
92 (57.5) |
5 (45.4) |
10 (90.9) |
0 (0.0) |
57 (64.0) |
77 (57.8) |
||||
|
Sharing of Toothbrush/Chewing Stick |
||||||||||
|
Yes |
10 (6.3) |
2 (18.1) |
2.87a (0.09) |
1 (9.1) |
0.16a (0.68) |
0 (0.0) |
3 (3.3) |
0.13a (0.71) |
5 (3.7) |
3.74a (0.05)* |
|
No |
150 (93.7) |
9 (81.8) |
10 (90.9) |
0 (0.0) |
86 (96.6) |
128 (96.2) |
||||
|
Sharing of Towel/ Underwear |
||||||||||
|
Yes |
2 (1.3) |
1 (9.1) |
5.88a (0.01)* |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0 (0.0) |
2.53a (0.11) |
1 (0.8) |
1.48a (0.22) |
|
No |
158 (93.7) |
10 (90.9) |
11 (100) |
0 (0.0) |
89 (100) |
132 (99.2) |
||||
|
Intravenous Drug User |
||||||||||
|
Yes |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
||||
|
No |
160(100) |
11 (100) |
11 (100) |
0 (0.0) |
89 (100) |
133 (100) |
||||
|
Tatoo/ Incision |
||||||||||
|
Yes |
3 (1.9) |
0 (0.0) |
0.15a (0.69) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
3 (2.3) |
0.64a (0.42) |
|
No |
157 (98.1) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
88 (98.9) |
130 (97.7) |
||||
|
Dental Procedure |
||||||||||
|
Yes |
4 (2.5) |
0 (0.0) |
0.77a (0.37) |
0 (0.0) |
0.30a (0.58) |
0 (0.0) |
0 (0.0) |
1.55a (0.21) |
1 (0.8) |
0.16a (0.68) |
|
No |
156(98.7) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
89 (100.0) |
132 (99.2) |
||||
|
Surgery |
||||||||||
|
Yes |
3 (1.9) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
0.22a (0.63) |
0 (0.0) |
1 (1.1) |
0.61a (0.43) |
3 (2.3) |
0.64a (0.42) |
|
No |
157 (98.1) |
11 (100.0) |
11 (100.0) |
0 (0.0) |
88 (98.9) |
130 (97.7) |
||||
|
Tribal Mark |
||||||||||
|
Yes |
93 (58.1) |
2 (18.2) |
0.30a (0.58) |
4 (36.4) |
2.29a (0.13) |
0 (0.0) |
45 (50.6) |
3.41a (0.06) |
79 (59.4) |
3.25a (0.07) |
|
No |
67 (41.9) |
9 (81.8) |
7 (63.6) |
0 (0.0) |
44 (49.4) |
54 (40.6) |
||||
|
Ear/Nose Piercing |
||||||||||
|
Yes |
67 (41.9) |
5 (45.4) |
0.77a (0.37) |
6 (54.5) |
4.61a (0.03)* |
0 (0.0) |
41 (46.1) |
9.85a (0.05)* |
51 (38.3) |
0.09a (0.76) |
|
No |
93 (58.1) |
6 (54.5) |
|
5 (45.4) |
|
0 (0.0) |
48 (53.9) |
|
82 (61.6) |
|
p-value significant at ≤0.05 and represented with *; p-value insignificant at ≥0.05; confidence interval (CI) at 95%.
References
- Nokhodian Z, Yaran M, Adibi P, Kassaian N, Meshkati M et al. (2014) Seroprevalence of hepatitis B markers among incarcerated intravenous drug users. J Res Med Sci 1: S13-S16. [Crossref]
- Center for Disease Prevention and Control (2015) Hepatitis B. Epidemiology and Prevention of Vaccine Preventable Diseases. Pink Book 149-174.
- Keten D, Ova ME, Keten HS, Keten A, Gulderen E et al. (2016) The Prevalence of hepatitis B and C among prisoners in Kahramanmaras, Turkey. Jundishapur J Microbiol 9: e31598. [Crossref]
- Salem F, Hekmat S, Aghasadeghi MR, Javadi F, Gholami H et al. (2013) Prevalence and Risk Factors of Hepatitis B Virus Genotype D Amongst Inmates in Alborz Province, Iran: A Cross-Sectional Survey. Jundishapur J Microbiol 6: e10221.
- Schweitzer A, Horn A, Mikolajczyk RT, Krause G, Ott JJ (2015) Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 386:1546-1555. [Crossref]
- World Health Organization (2018) Hepatitis B Fact Sheet.
- Yakasai IA, Ayyuba R, Abubakar IS, Ibrahim SA (2012) Sero-prevalence of Hepatitis B Virus Infection and its Risk factors among Pregnant Women Attending Antenatal Clinic at Aminu Kano Teaching Hospital, Kano, Nigeria. J Basic Clin Reprod Sci 1: 49-54.
- Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL (2015) Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: A systematic review and meta-analysis. Niger J of Clin Pract 18: 163-172. [Crossref]
- Gboeze AJ, Ezeonu PO, Onoh RC, Ukaegbe CI, Nwali MI. Knowledge and awareness of HBV infection among pregnant women in Abakaliki Nigeria. J Hepatitis Res 2: 1-4.
- Iklaki CU, Emechebe CI, Ago BU, Njoku CO (2015) Seroprevalence of Hepatitis B Infection and Its Risk Factors among Women Admitted for Delivery in UCTH, Calabar, Nigeria. Br J Med Med Res 4: 324-333.
- Bzowej NJ (2010) Hepatitis B therapy in pregnancy. Curr Hepatitis Rep 9: 197-204. [Crossref]
- Ugwuja E, Ugwu N (2008) Seroprevalence of Hepatitis B Surface Antigen and Liver Function Tests among Adolescents in Abakaliki, Southeastern Nigeria. Internet J Trop Med 6: 1-6.
- National Population Commission (2018) State Population. 2006.
- Naing L, Winn T, Rusli BN (2006) Medical Statistics: Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci 1: 9-14.
- Araoye MO (2004) Sample size determination. Res Methodol Statis Health Social Sci.
- Abulude OA, Ahmed I, Sadisu FU (2017) Assessment of Hepatitis B Viral Infection as a Predictor of Hepatic Enzymes and Compounds Alteration among Antenatal Patients. Med Sci (Basel) 5. [Crossref]
- Ndams IS, Joshua IA, Luka SA, Sadiq HO (2008) Epidemiology of hepatitis B infection among pregnant women in Minna, Nigeria. SWJ 3: 5-8.
- Mbaawuaga EM, Enenebeaku MNO, Okopi JA, Damen JG (2008) HBV infection among pregnant women in Markudi, Nigeria. Afr J Biomed Res 11: 155-159.
- Bayo P, Ochola E, Oleo C, Mwaka AD (2014) High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in northern Uganda. Br Med J Open 4: e005889. [Crossref]
- Chen S, Ravallion M (2008) The developing world is poorer than we thought, but not less successful in the fight against poverty. Washington, DC, World Bank: Policy Research Working Paper No. 4703.
- Ndako JA, Echeonwu GON, Nwankiti OO, Onovoh EM (2012) Hepatitis B virus sero-prevalence among pregnant females in northern Nigeria. J Res Med Sci 3: 129-133.
- Afzali H, Heravi MM, Moravveji SA, Poorrahnama M (2015) Prevalence of Hepatitis B Surface Antigen in Pregnant Women in Beheshti Hospital of Kashan, Isfahan. Iran Red Crescent Med J 7: 1-5. [Crossref]
- Olaitan AO, Zamani LG (2010) Prevalence of hepatitis B virus and hepatitis C virus in ante-natal patients in Gwagwalada-Abuja, Nigeria. Rep Opinion 7: 48-50.
- Abongwa LE, Kenneth P (2016) Assessing Prevalence and Risk Factors of Hepatitis B Surface Antigen amongst Pregnant Women attending Antenatal Clinic in the Northwest Region of Cameroon. Eur J Res Med Sci 4: 32-43.
- Opaleye OO, Salami S, Familua F, Olowe OA, Ojurongbe O et al. (2014) Seroprevalence of Hepatitis B Surface Antigen and Antibody among Pregnant Women Attending a Tertiary Health Institution in Southwestern Nigeria. IOSR J Dent Med Sci 13: 2279-0861.
- Pennap GR, Muazu IF, Fatima M (2015) Parallel and Overlapping Hepatitis B and C Virus Infection among Pregnant Women Attending Antenatal in a Rural Clinic in Northern Nigeria. Int J Curr Microbiol APPL SCI 5: 16-23.
- Oladeinde BH, Omoregie R, Oladeinde OB (2013) Prevalence of HIV, HBV, and HCV infections among pregnant women receiving antenatal care in a traditional birth home in Benin City, Nigeria. Saudi J Health Sci 2: 113-117.
- Olokoba AB, Salawu FK, Danburam A, Olokoba LB, Midala JK et al. (2011) Hepatitis B virus infection amongst pregnant women in North-Eastern Nigeria- A call for action. Niger J Clin Pract 14: 10-13. [Crossref]
- Liang TJ (2009) Hepatitis B: The Virus and Disease. Hepatology 49: 13-21. [Crossref]
