Persistence of Positive RT-PCR for SARS-CoV-2 in an Early Allogenic Stem Cells Transplantation Recipient: A Case Study
A B S T R A C T
The new coronavirus SARS-CoV-2 has rapidly spread over the world causing the disease by WHO called COVID-19, is the defining global health crisis of our time and the greatest threat we have faced during this century [1]. According to the World Health Organizations (WHO) data, in February 2021, almost one hundred fifteen million of the population has been infected with more than two million deaths [2]. To date, in the absence of any specific treatment, our knowledge about this disease remains very limited and is subject to rapid change. This virus represents a serious danger for patients with hematologic malignancies due to myeloablative conditioning and immunosuppressive treatments. People receiving chemotherapy with compromised immune systems and complications after stem cell transplant have an increased risk for infection [3]. COVID-19 infection may complicate clinical symptoms with higher risk of respiratory distress [4]. Actually, the experience in the management of COVID-19 infection in the stem cell transplant recipients is limited.
Keywords
COVID-19, SARS-CoV-2, bone marrow transplantation, conditioning, post-transplant cyclophosphamide
Introduction
A novel coronavirus named SARS-CoV-2 of a zoonotic origin emerged at the end of the last year and the disease called Coronavirus Disease 2019 (COVID-19) started spreading worldwide [1]. COVID-19 infection has spread very rapidly in the population of several countries. The time from exposure to symptom development is between 2 and 14 days. Symptoms vary from no or mild symptoms of an upper respiratory infection to severe resulting in the need for intensive care and death from Acute Respiratory Distress Syndrome (ARDS) [5]. Increasing age and the presence of comorbidities, such as hypertension, cardiovascular disease, diabetes, and pulmonary disease, are reported risk factors for severe disease and mortality [6, 7]. Patients, who develop more severe symptoms including respiratory failure, often progress during the 2nd week after the start of symptoms and it is believed that this is to a great extent due to an immune reaction in the lower airways [8]. Cancer is considered a risk factor for COVID-19 infection (1%), and people receiving chemotherapy with compromised immune systems and complications after stem cell transplant have an increased risk for infection, with very few cases reported in hematology-oncology, but with no data related to bone marrow transplant [3, 9].
Therefore, the management of patients transplanted during a pandemic is very complex. To overcome this crisis, some recommendations and emergency measures have been developed by scientific societies like the European Society for Blood and Bone Marrow Transplantation [1]. Their guidelines are established according to the coronavirus high contagion rate in Europe, however currently there are not approved treatment options in Europe and there is no available vaccine, further patients with cancer had poorer outcomes from COVID-19 [1, 9]. Actually, the experience in the management of COVID-19 infection in the stem cell transplant recipients is limited. Here we report 1 COVID‐19 case with prior history of allogeneic bone marrow transplantation.
Case Report
We present a case of a 57-year-old male, without significant comorbidities, who was diagnosed in September 2019 with Acute Myeloid Leukemia (AML) with recurrent genetic abnormalities due to t (9; 11) (p21.3; q23.3); MLLT3-KMT2A, with adverse cytogenetic prognosis according to the European leukemia Net, he received management with a 7+3 induction protocol, with a complete morphological response, and continuing consolidation with high doses of cytarabine and a subsequently allogeneic stem cells transplantation from a related histocompatible donor (Brother HLA 10 /10) was done at January 25 of 2020, conditioning with Fludarabine plus TBI (Total Body Irradiation) was used. He receives prophylaxis for graft-versus-host disease (GVHD), with cyclosporine, prior to the reinfusion of hematopoietic progenitors, a myeloid graft was obtain on day +14 and platelet graft on day +15, he subsequently received cyclophosphamide, as a strategy against GVHD, he was sent home with oral cyclosporine as an immunosuppressant, additionally acyclovir, posaconazole and trimethoprim-sulfa, as infectious prophylaxis, there were not complications immediately. In the follow-up at day +80 (13.04.2020), acute GVHD grade 2 was diagnosed, requiring management with systemic steroids, which resulted in a complete response, continued management with prednisolone 50 mg per day and prophylaxis with Acyclovir and Posaconazole.
He was admitted to the emergency department on April 27 of 2020 (day + 92 post-transplant) due to 8 days of asthenia, adynamia, generalized weakness and fever of 38.3℃, the patient denied contact with respiratory symptomatic persons or with suspected or confirmed SARS-CoV-2 cases; Upon admission, he was hemodynamically stable without evidence of respiratory distress, paraclinical studies shows pancytopenia with severe neutropenia, INL <3, hyperferritinemia in addition to elevation of D Dimer, elevated IL6, C-reactive protein, and positive procalcitonin (Table 1).
Table 1: Relevant results of blood tests obtained during the evolution of the COVID-19 infection.
|
Fecha |
WBC |
Neu |
Lymp |
Mono |
Hb |
Hcrt |
Pla |
Ferritine |
D dim |
IL6 |
PA/FI |
N/L |
CD3/CD4 |
CD3/CD8 |
|
27.04.2020 |
800 |
110 |
380 |
310 |
8.8 |
26.4 |
37000 |
7650 |
- |
19.8 |
362 |
0.29 |
|
|
|
04.05.2020 |
4230 |
3570 |
320 |
100 |
11.2 |
32.6 |
20000 |
17139 |
- |
326 |
81 |
11.1 |
|
|
|
06.05.2020 |
4090 |
3430 |
310 |
130 |
10 |
31.2 |
14000 |
22117 |
2.25 |
338 |
283 |
11 |
|
|
|
08.05.2020 |
5230 |
4680 |
200 |
110 |
8.8 |
26.7 |
26000 |
21743 |
2.56 |
- |
227 |
23.4 |
|
|
|
14.05.2020 |
17700 |
15500 |
540 |
640 |
9.5 |
27.9 |
15000 |
7367 |
1.8 |
- |
175 |
28.7 |
|
|
|
17.05.2020 |
7550 |
6710 |
130 |
280 |
7.9 |
23.7 |
14000 |
- |
- |
- |
227 |
51.6 |
|
|
|
21.05.2020 |
6080 |
5490 |
200 |
230 |
9.9 |
28.3 |
14000 |
6580 |
- |
- |
91 |
27.4 |
|
|
|
26.05.2020 |
4140 |
3620 |
370 |
110 |
7.1 |
21.9 |
22000 |
8307 |
- |
- |
198 |
9.78 |
|
|
|
28.05.2020 |
2310 |
2060 |
100 |
110 |
7.2 |
21.3 |
12000 |
- |
- |
- |
147 |
20.6 |
|
|
|
30.05.2020 |
7830 |
7210 |
180 |
320 |
7.1 |
22.2 |
13000 |
- |
3.3 |
- |
219 |
40 |
|
|
|
03.06.2020 |
5740 |
5110 |
190 |
340 |
9.2 |
27.7 |
17000 |
4845 |
- |
- |
243 |
26.8 |
|
|
|
08.06.2020 |
2550 |
2130 |
250 |
140 |
7.4 |
23.3 |
17000 |
5052 |
- |
- |
322 |
8.5 |
|
|
|
14.06.2020 |
3460 |
2730 |
390 |
310 |
7.7 |
23.0 |
27000 |
- |
- |
- |
213 |
7 |
|
|
|
20.06.2020 |
4920 |
3870 |
480 |
320 |
6.6 |
21 |
30000 |
- |
- |
- |
179 |
8.06 |
|
|
|
26.06.2020 |
4230 |
3000 |
490 |
510 |
7.6 |
23.8 |
52000 |
- |
- |
- |
306 |
6.12 |
|
|
|
30.06.2020 |
4470 |
3200 |
630 |
370 |
7.2 |
22.4 |
51000 |
- |
- |
- |
283 |
5.08 |
|
|
|
04.07.2020 |
5600 |
3870 |
960 |
430 |
7.4 |
23.1 |
59000 |
- |
- |
- |
|
4.03 |
|
|
|
15.07.2020 |
|
|
|
|
|
|
|
|
|
|
|
|
261 |
626 |
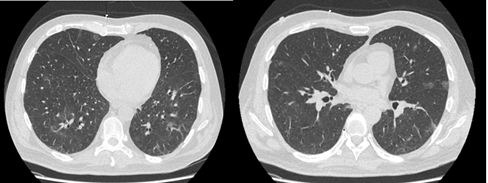
High-resolution computed tomography of the chest was performed, and describes the presence of multifocal ground glass radiopacities, with subpleural and centrilobular localization, and the presence of centrilobular nodules (Figure 1). He was managed with cefepime and prophylaxis with trimethoprim/sulfamethoxazole, acyclovir, and posaconazole and a SARS-CoV-2 RT PCR was requested, which was positive, leading to the diagnosis of Acute respiratory infection due to SARS- -CoV-2, management with Hydroxychloroquine plus Azithromycin was initiated, according to institutional protocol (receiving this combination for a total of 7 days) and vigilance in the intensive care unit was indicated, due to high risk of respiratory complications, additionally human granulocyte-colony stimulating factor was administered due to the evidence of severe neutropenia that was interpreted as a spinal brake.
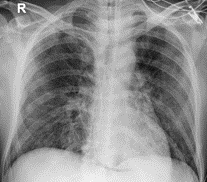
The course of the disease was torpid, on the 7th day of hospitalization (04.05.2020) he presented signs of respiratory distress with severe hypoxemia and worsening of pulmonary infiltrates documented in imaging studies (Figure 2), bacterial overt infection by methicillin resistant Staphylococcus aureus was documented by a nasopharyngeal swab, requiring orotracheal intubation, prone invasive mechanical ventilation and modification of the antibiotic coverage, continuing management with meropenem plus ceftaroline. Given the high levels of IL6, the addition of tocilizumab was considered, however due to the evidence of overt infection this possibility was ruled out. His general condition worsens presenting septic shock with severe signs of hypoperfusion and a marked inflammatory response was evidenced (Table 1), requiring vasoactive, inotropic support, the addition of caspofungin and the modification of the antibiotic regimen, suspending meropenem and continuing with ceftazidime/avibactam.
Figure 1: High-resolution computed tomography of the chest showing multifocal ground glass radiopacities, with subpleural and centrilobular localization.
Figure 2: Chest X-ray showing bilateral reticular interstitial opacities that have increased in the right pulmonary field and peripheral ground glass opacity occupying the middle region of the left pulmonary field.
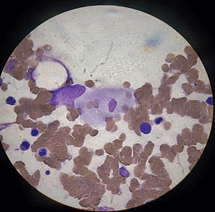
On the 11th day of hospitalization (08.05.2020), given the persistence of pancytopenia and hyperferritinemia, a bone marrow aspiration was performed with flow cytometry where no myeloid blasts were detected in 9,147,296 events, however the myelogram shows findings compatible with hemophagocytosis (Figure 3).
Figure 3: Myelogram (x100) Showing hemophagocytosis. Macrophage engulfing red blood cells.
During the following days, his evolution was poor, with refractory hypoxemia that doesn't improve with pronation and relaxation maneuvers, as well as septic shock and persistent hypoperfusion. Further a perianal abscess was documented, being managed with metronidazole and presenting spontaneous drainage the following days. Later, secondary to barotrauma due to the high pressures needed for ventilation, an extensive pneumomediastinum and a subcutaneous thoraco-abdominal and inguinal emphysema was detected (12.05.2020). On day 20 of hospitalization (17.05.2020), after improvement of his respiratory condition and hemodynamic status, extubation was achieved, continuing management with a high-flow nasal cannula, however, due to an hyperactive delirium, the nasogastric tube was accidentally removal by the patient resulting in bronchial aspiration that derived in a ventilatory failure, requiring re-intubation and restarting of the vasoactive support with the addition of methylene blue due refractory vasodilated septic shock.
After the last event described the hemodynamic parameters began to improve allowing suspension of the vasoactive support, however, given the prolonged intubation and the failed extubation episode, a tracheostomy was performed. Further, he presented necrotic sacral eschar secondary to a state of hypoperfusion and prostration, requiring debridement plus washing and placement of a vacuum-assisted closure (VAC) system on 06.07.2020. During the treatment, several SARS-CoV-2 RT PCR were performed, persisting positive (por more than 8 weeks) with IgM for SARS-CoV-2 positive but IgG initially Negative (06.06.2020) with positivization 6 week later at 12.06.2020.
Despite the persistence of Positive RT PCR, the patient presents a satisfactory evolution, ventilatory support a supplemental oxygen has been suspended, the management of sacral eschar continue by the enterostomal therapy nursing staff, without the need of VAC system, he is on a respiratory, physical and nutritional rehabilitation plan and has a hemogram with moderate anemia and thrombocytopenia without transfusion requirement and without alteration of the white line. There is no evidence of AML relapse, (100% donor chimerism and negative bone marrow cytometry in evaluated events) graft rejection or cytomegalovirus reactivation (all viral loads for cytomegalovirus were negative).
Discussion
The management of COVID-19 remains uncertain in the general population, and in the population of transplant patients the information is even more uncertain and unknown, according to the latest reports, comorbidities increase the risk of severe pneumonia in patients with confirmed infection by COVID-19, however, a history of allogeneic bone marrow transplantation has not yet been considered among the risk factors [7]. Infection with this coronavirus can cause a sustained cytokine response (called cytokine storm), leading to a high incidence of immune disorders and mortality [10]. Lymphocytes and their subsets play an important role in maintaining the function of the immune system. As with immune diseases and other infectious diseases, virus infection can also lead to dysregulation in the levels of lymphocyte subsets [11]. These cells are involved in humoral and cytotoxic immunity against viral infection.
The age of this patient is relatively younger than the age reported in the literature, related to worse outcomes, therefore, it may be reasonable to think that the history of allogenic bone marrow transplantation may play an important role in the progression of COVID-19 pneumonia to ventilatory failure [8]. The function of the graft was affected at first, possibly secondary to the bone marrow brake typical of an infection, however with the administration of human granulocyte-colony stimulating factor, the leukocyte count improved, at the expense of neutrophils and monocytes, however it presented persistent lymphopenia, it is important to note that the number of T lymphocytes, both CD4+ and CD8+, were decreased in this patient, as expected in early allogeneic transplantation in the first 3-6 months with histocompatible HLA 10/10 [12]. Regarding immunosuppressive agents, the patient was receiving tacrolimus as an immunosuppressive drug prior to COVID-19 infection.
The patient presents a late immune reconstitution, which means that his humoral response is not yet reconstituted, and the development of a germinal center reaction depending on follicular CD4+ lymphocytes and B lymphocytes did not develop completely. We do not know if this is a protective factor in severe infection by COVID-19, and if it is related to a better modulated inflammatory reaction in these types of patients, which is represented in the persistence of the RT PCR for SARS-CoV-2 persistently positive even after 60 days of having presented the onset of symptoms, while the latest reported viral load found in the literature was 37 days [13].
Conclusion
Here we report the case of a patient with severe infection by COVID-19, recipient of an allogeneic bone marrow transplant, with a favourable evolution that raises the possibility of incomplete immune reconstitution as a protective factor for mortality due to COVID-19 infection.
Author Contributions
All authors were involved in writing, reading, and editing the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of Data and Materials
All data analysed during this study are included in this published article and were derived from the archived medical records of Fundación Valle del Lili University Hospital, Santiago de Cali, Colombia.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Written informed consent was obtained from the patients’ legal guardians for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing Interests
None.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 06, Mar 2021Accepted: Tue 16, Mar 2021
Published: Fri 02, Apr 2021
Copyright
© 2023 Jaramillo E. Francisco Javier. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.TCR.2021.01.02
Author Info
Hernandez T. Andres Felipe Castaño Q. Santiago Celin V. Daniel Eduardo Jaramillo E. Francisco Javier
Corresponding Author
Jaramillo E. Francisco JavierDepartment of Hematology and Bone Marrow Transplantation, Hospital Universitario Fundacion Valle del Lili, Santiago de Cali, Colombia
Figures & Tables
Table 1: Relevant results of blood tests obtained during the evolution of the COVID-19 infection.
|
Fecha |
WBC |
Neu |
Lymp |
Mono |
Hb |
Hcrt |
Pla |
Ferritine |
D dim |
IL6 |
PA/FI |
N/L |
CD3/CD4 |
CD3/CD8 |
|
27.04.2020 |
800 |
110 |
380 |
310 |
8.8 |
26.4 |
37000 |
7650 |
- |
19.8 |
362 |
0.29 |
|
|
|
04.05.2020 |
4230 |
3570 |
320 |
100 |
11.2 |
32.6 |
20000 |
17139 |
- |
326 |
81 |
11.1 |
|
|
|
06.05.2020 |
4090 |
3430 |
310 |
130 |
10 |
31.2 |
14000 |
22117 |
2.25 |
338 |
283 |
11 |
|
|
|
08.05.2020 |
5230 |
4680 |
200 |
110 |
8.8 |
26.7 |
26000 |
21743 |
2.56 |
- |
227 |
23.4 |
|
|
|
14.05.2020 |
17700 |
15500 |
540 |
640 |
9.5 |
27.9 |
15000 |
7367 |
1.8 |
- |
175 |
28.7 |
|
|
|
17.05.2020 |
7550 |
6710 |
130 |
280 |
7.9 |
23.7 |
14000 |
- |
- |
- |
227 |
51.6 |
|
|
|
21.05.2020 |
6080 |
5490 |
200 |
230 |
9.9 |
28.3 |
14000 |
6580 |
- |
- |
91 |
27.4 |
|
|
|
26.05.2020 |
4140 |
3620 |
370 |
110 |
7.1 |
21.9 |
22000 |
8307 |
- |
- |
198 |
9.78 |
|
|
|
28.05.2020 |
2310 |
2060 |
100 |
110 |
7.2 |
21.3 |
12000 |
- |
- |
- |
147 |
20.6 |
|
|
|
30.05.2020 |
7830 |
7210 |
180 |
320 |
7.1 |
22.2 |
13000 |
- |
3.3 |
- |
219 |
40 |
|
|
|
03.06.2020 |
5740 |
5110 |
190 |
340 |
9.2 |
27.7 |
17000 |
4845 |
- |
- |
243 |
26.8 |
|
|
|
08.06.2020 |
2550 |
2130 |
250 |
140 |
7.4 |
23.3 |
17000 |
5052 |
- |
- |
322 |
8.5 |
|
|
|
14.06.2020 |
3460 |
2730 |
390 |
310 |
7.7 |
23.0 |
27000 |
- |
- |
- |
213 |
7 |
|
|
|
20.06.2020 |
4920 |
3870 |
480 |
320 |
6.6 |
21 |
30000 |
- |
- |
- |
179 |
8.06 |
|
|
|
26.06.2020 |
4230 |
3000 |
490 |
510 |
7.6 |
23.8 |
52000 |
- |
- |
- |
306 |
6.12 |
|
|
|
30.06.2020 |
4470 |
3200 |
630 |
370 |
7.2 |
22.4 |
51000 |
- |
- |
- |
283 |
5.08 |
|
|
|
04.07.2020 |
5600 |
3870 |
960 |
430 |
7.4 |
23.1 |
59000 |
- |
- |
- |
|
4.03 |
|
|
|
15.07.2020 |
|
|
|
|
|
|
|
|
|
|
|
|
261 |
626 |
References
1. Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C et al. (2020) The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant 55: 2071-2076. [Crossref]
2. World health Organization (2020) Coronavirus disease 2019 (COVID-19) Dashboard. 2020. Accessed 7 July 2020.
3. Wingard JR, Hsu J, Hiemenz JW (2011) Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am 25: 101-116. [Crossref]
4. Allareddy V, Roy A, Rampa S, Lee MK, Nalliah RP et al. (2014) Outcomes of stem cell transplant patients with acute respiratory failure requiring mechanical ventilation in the United States. Bone Marrow Transplant 49: 1278-1286. [Crossref]
5. Zhou F, Yu T, Du R, Fan G, Liu Y et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054-1062. [Crossref]
6. Yang J, Zheng Y, Gou X, Pu K, Chen Z et al. (2020) Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis 94: 91-95. [Crossref]
7. Wu C, Chen X, Cai Y, Xia J, Zhou X et al. (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180: 934-943. [Crossref]
8. Wang D, Hu B, Hu C, Zhu F, Liu X et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061-1069. [Crossref]
9. Liang W, Guan W, Chen R, Wang W, Li J et al. (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21: 335-337. [Crossref]
10. Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529-539. [Crossref]
11. Chan MH, Wong VW, Wong CK, Chan PKS, Chu CM et al. (2004) Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J Intern Med 255: 512-518. [Crossref]
12. Qin C, Zhou L, Hu Z, Zhang S, Yang S et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71: 762-768. [Crossref]
13. Zhou F, Yu T, Du R, Fan G, Liu Y et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054-1062. [Crossref]
