Persistence of Glenohumeral Subluxation is Correlated with Prolonged Existence of Crossed Cerebellar Diaschisis in a Hemiplegic Stroke Survivor: A Pilot Study
A B S T R A C T
Glenohumeral Subluxation (GHS) is one cause of shoulder pain after stroke. The greater the distance of GHS, the higher is the chance of rotator tendonitis or tearing of the tendon, causing limited motions and excruciating pain. Cross Cerebellar Diaschisis (CCD), a reduction of blood flow in the contralateral cerebellum after the supratentorial stroke, is detectable by a brain perfusion scan, and it has marked impacts on functional outcomes after stroke. We presented here a case on hemiplegic stroke. CCD of the patient persisted for 7 months without improvements. The patient underwent measurement of GHS and Single Photon Emission Computed Tomography to confirm the characteristic relationship. The patient’s GHS persisted for a prolonged period of time during which the acromiohumeral distance was longer than those of the general CCD-free stroke. Together with persisted CCD, the persistence of GHS was correlated with a prolonged CCD, which is presumably one sign of motor deficits associated with CCD.
Keywords
Glenohumeral subluxation, crossed cerebellar diaschisis, acromiohumeral distance, single photon emission computed tomography, complex regional pain syndrome
Introduction
Patients after stroke often display symptoms like aphasia, half neglect, limb hemiplegia, abnormal sensation, cognitive impairment, imbalance, and brainstem or cerebellar dysfunction. Vuagnat & Chantraine reported that, in stroke patients, 16% to 84% experience shoulder pain as one common complication of stroke [1]. Regarding causes of shoulder pain, reports are related to lesions of the rotator cuff tendons, central post-stroke pain, lack of sensibility, unilateral neglect, spasticity, reflex sympathetic dystrophy, and inferior-anterior subluxation of the head of the humerus [1]. Glenohumeral Subluxation (GHS) is common in hemiplegic patients with shoulder pain [2]. GHS, closely related to upper limb function, is found most often in stroke patients [2]. Although GHS is not typically accompanied by pain initially, as the patient continues in the flaccid stage or with injuries in soft tissues, pain may appear over an area half to two-finger widths at the shoulder joint [3]. Yang et al. treated such patients with hemiplegic stroke using magnetic stimulation and found a significant drop in the bilateral differences of acromiohumeral distance (AHD) (t = 8.375, P <0.01) [4]. In a recent study on functional electrical stimulation for treating shoulder pain in stroke patients, Karaahmet et al. found a positive correlation between GHS and pain (P= 0.022) [5].
Crossed Cerebellar Diaschisis (CCD) is a condition involving a reduced blood flow and reduced oxygen metabolism in the contralateral cerebellum after a supratentorial stroke [6]. Techniques measuring cerebral oxygen metabolism and brain glucose metabolism have been used to study CCD [7]. In stroke patients, CCD has some reported impacts on functional outcomes like psychologic performance, aphasia, postural asymmetry [8-10]. However, the relationship between CCD and GHS remained unexplored. Here we present a case involving hemiplegic stroke, where we found that the persistence of GHS was correlated with a prolonged CCD.
Case Report
A 52-year-old woman, upon waking up from a nap on 2018/04/12, suddenly suffered from an unsteady gait and reduced body sensation on her right side. She was soon sent to a local medical center. Her consciousness was clear, pupils: 2+/2+, with vital signs of BP: 132/81, HR: 80, RR: 22, BT: 36. A neurological examination revealed manual muscle testing (MMT) as 5 in left-sided limbs, 3 in right upper extremity, and 2 in the right lower extremity, and an initial NIHSS of 5. The initial computed tomography (CT) revealed no signs of intracerebral hemorrhage, and she was diagnosed with acute cerebral infarction in her left cerebral hemisphere and a right-side hemiplegia. Early next morning (4/13), her neurologic deficits exaggerated. A brain CT was quickly performed, revealing a massive acute hematoma at her left frontoparietal lobe, leading to edema and subfalcine herniation. She underwent an emergent decompressive craniotomy, and her intracerebral pressure was monitored. The surgical operation involved cranioplasty and ventriculoperitoneal shunting. The patient was transferred to the Intensive Care Unit for post-operative care. She was then housed in an ordinary ward where she subsequently underwent inpatient rehabilitation. The patient was finally discharged and received inpatient rehabilitation in several hospitals during the following months.
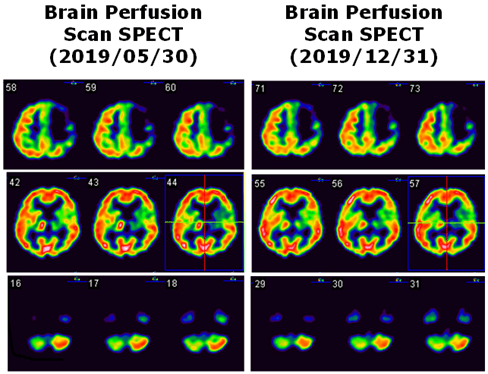
Due to persistent right hemiplegia and aphasia, she visited our hospital for the first time on 2019/05/29. On examination, a remarkable pain, with allodynia, was noted at her right upper extremity. The right hand and palm appeared clearly swollen. There was a gap at the shoulder joint of approximately half- to two-finger widths. Musculoskeletal sonography excluded the possibility of partial/complete tear of the rotator cuff. Single Photon Emission Computed Tomography (SPECT) with 99mTc Ethyl Cysteinate Dimer (ECD) was arranged to evaluate cerebral blood flow. Relatively reduced radioactivity was found in the left temporal, left medial frontal regions, left striatum, and left thalamus, as well as the right cerebellum (Figure 1). The impression of CCD was noted on her right side. Of interest, we also noted an increased uptake at the right thalamus (Figure 1). X-ray films of the shoulder joint were analysed on 2019/07/08, showing clear signs of GHS. An AHD of 21.6 mm was measured on the right side and 10.8 mm on the left side (Figure 2).
Figure 1: The Tc-99m ECD brain perfusion SPECT study of our case. Left panel shows the first images with decreased radioactivity in the left frontoparietal (upper), left striatum and thalamus (middle), and right cerebellum (lower), e.g., crossed cerebellar diaschisis in the right cerebellum. Right panel is the second images showing identical uptake in the left panel, except that the uptake of the right thalamus changes from increased uptake to normal uptake.
During the following 6 to 7 months, the patient experienced right-sided hemiplegia while still able to walk slowly by herself under supervision. Her pain in the right shoulder nevertheless persisted, even after aggressive rehabilitation for 5 months, including steroid injections to the right GH. When her right arm was passively pulled back or raised horizontally over 80⁰, she felt pain. The outward extension was >70⁰, and the backward was >20⁰. Shoulder sonography still revealed negative. The AHD was measured 19.6 mm on the right and 8.4 mm on the left (Figure 2). The second Brain ECD-SPECT showed CCD remaining on the right side, but not the prior hyperperfusion in the right thalamus (Figure 1).
Figure 2: Plain X-ray films of our patient showing the shoulder joint. Values of AHD measured by radiographic software (Smartiris) are indicated on the images, and GHS is remarkably obvious on the affected side (right) (see left upper and left lower). The first images show an AHD in the affected side (right) of 21.6 mm, compared with 10.8 mm on the other side (upper panel). The second images show AHD of 19.6 mm in the affected side (right) vs. 8.4 mm on the left side (upper panel). Nearly 5 months later, the difference of AHD on the affected side changed minimally (by only 2 mm), which likely was closely correlated with persistent CCD. AHD: acromiohumeral distance; CCD: crossed cerebellar diaschisis; GHS: glenohumeral subluxation.
Discussion
For two decades, various methods have been used to study the impact of CCD on stroke-related symptoms like postural asymmetry [1]. It is generally believed that CCD is caused by an interruption of either the cortico-ponto-cerebellar pathway or the dentate-rubro-thalamo-cortical pathway [11, 12]. However, no study has been done to explore the close relationship between GHS and CCD. One possible reason for that is both GHS and CCD are considered symptoms of stroke, and they occur together. Based on the clinical presentation, we hypothesized that if GHS incorporates CCD, CCD will exacerbate GHS. In general, the average AHD is 9 mm. An AHD >9.5mm indicates the presence of GHS [13]. If the AHD is larger, it may be due to dislocation. The AHD, from calculations in our case, was 21.6 mm (1st film) and 19.6 mm (2nd film) (Figure 2). We found that CCD had caused the patient's right arm to experience a downward displacement more severely than those patients without CCD, having a mean AHD of 16.4 to 18.0 mm. This patient with hemiplegic stroke demonstrated the close relationship between a persistent GHS and a prolonged CCD.
Traditionally, GHS is considered due to three causes. First, because the tension on shoulder-supporting muscles is low, there is little muscle activity. Gravity, therefore, pulls the arm down. Second, the joint capsule of the glenohumeral joint is loosened. Third, because the range of shoulder joint motion is the greatest amongst all joints in the body, any incorrect posture may cause the arm to be pulled and the shoulder dislocated. These three causes lead to the scapular bone being moved away from the joint cavity. It is likely that stroke produces hemiplegia, leading to reduced muscle tensions around the joint, destabilizing the joint. The greater the distance produced, the higher the chance of rotator tendonitis, tearing of the tendon and accumulation of synovial fluid. If this condition goes undetected early, and is improperly handled, it may cause shoulder pain and limit shoulder movements. However, our patient did not have problems with her shoulder joint prior to her stroke episode, and a bigger AHD, which persists far from the common length measured in a hemiplegic shoulder is not a good sign.
The relationship between CCD and GHS could be better understood given first a good background knowledge of the Complex Regional Pain Syndrome (CRPS). CRPS is a neuropathic pain disorder that develops as a response to traumatic lesions or nerve damages [14]. Patients with shoulder-hand syndrome likely have GHS at higher incident rates than those of other hemiplegic patients [15]. Interestingly, our patient had upper limb pain/allodynia and swelling of the hand, all of which are consistent with an acute onset of CRPS. In addition, the patient’s initial SPECT scan revealed increased uptakes at the right thalamus, a brain structure known to be associated with acute onset CRPS [16]. CRPS can also occur simultaneously with CCD [17]. Our finding indicated the importance of the spino-reticulo-thalamic pathway, which goes upward ipsilaterally to the thalamus.
Serrati et al. used the Mathew and Orgogozo scales to study the relationship between CCD and clinical outcomes on Day 60 and reported that CCD in the early chronic stage is strongly associated with the neurologic outcome [18]. A recent study confirmed that CCD is associated with severe functional impairments, and that may adversely affect functional outcomes in cognitive function, ambulatory function, and ADL during the subacute rehabilitation phase of the stroke [19]. Our patient had a severe GHS. Additionally, her GHS condition also persisted together with AHD on a period of time longer than those of an average CCD-free stroke patient. Findings were consistent with the severity of GHS of our patient. Speculation has been made that CCD worsens shoulder joint problems after the stroke.
With respect to the pathophysiological mechanisms underlying CRPS and GHS, one could infer them based on the following two phenomena. First, the severity of CRPS is related to sensory disturbances and spasticity, as well as the severity and recovery of motor deficits [20]. An important factor in CRPS is the reorganization of the central nervous system, particularly affecting the primary somatosensory cortex [20]. One study reported a significant influence related to neglect and sensory impairment, which may lead to additional trauma or disturbed pain perception [21]. According to our case, we speculated that CRPS may result in sensory impairment, affecting the right limb and shoulder joint more seriously. The sympathetic nervous system can modulate the metabolic and hormonal regulation of bone and its mechanical micro-environment [22]. Second, chronic pain originating from musculoskeletal or neuropathic lesions is subject to central sensitization, a process that increases the responsiveness of nociceptive neurons in the CNS to normal or sub-threshold afferent inputs [23].
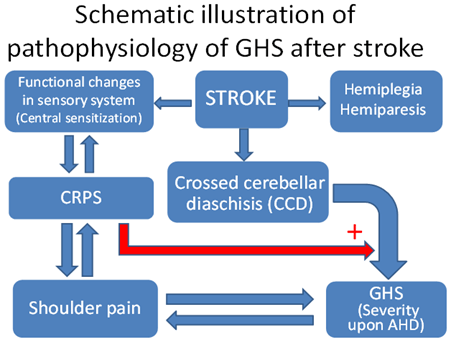
It is, therefore, reasonable to say that central sensitization worsens a patient’s GHS problem. The main cause of GHS in post-stroke patients is also associated with impaired sensory pathways, besides muscle fatigue. Another study pointed out that nerve injury could exacerbate the patient's shoulder injury. We speculated that CCD would worsen the GHS. We, therefore, assumed the affection of CRPS toward GHS, and that indirectly inferred the relationship between CCD and the severity of GHS. From the perspective of CRPS on the sensory system, we suspected that CRPS indirectly affects the severity of CCD and supporting a relationship between CCD and GHS is. The above arguments regarding the pathophysiology of GHS and CCD are illustrated in (Figure 3). In addition to its medical use, the AHD measurement is also helpful to physical therapists and sports coaches in deciding if their athletes can benefit from specific motion training. Normally, the AHD of healthy shoulders is 9±2 mm (range between 7 and 11 mm) [24]. With active shoulder abduction, trained athletes show a larger AHD compared with controls. Apparently, additional training exercises are aimed at expanding the subacromial space. This may be a sport-specific adaptation to prevent injuries [25].
Figure 3: Schematic illustration of the pathophysiology of GHS and CCD after stroke. CRPS: Complex Regional Pain Syndrome; GHS: Glenohumeral Subluxation; AHD: Acromiohumeral Distance.
GHS was assessed clinically using a linear measure of fingerbreadths between the acromion and the humeral head [26]. AHD has been studied in patients with rotator cuff disorder using radiograph plain films, magnetic resonance imaging, and musculoskeletal ultrasonography [4, 5, 27]. With respect to the reliability of radiological methods for measuring AHD in rotator cuff tendinopathy, a systematic review found strong evidence on the reliability of ultrasound, with moderate evidence on MRI and CT measures and conflicting evidence on radiographic methods. Results supported better reliability of ultrasound and CT or MRI in measuring AHD [28]. Because most researchers found high reproducibility of ultrasonographic measurements, some researchers recommended measuring AHD with ultrasonography as a diagnostic tool to confirm the severity of GHS in patients with post-stroke hemiplegia [29]. The reliability of AHD measurements using radiographs was, however, unsupported by the reviewed literature despite the simplicity of the radiographic approach [5, 27]. We agree with those recommendations, even though our patient was not tested with all the techniques recommended.
The limitations of our case report are described below. First, we did not use ultrasonography as a measurement. Second, we found the AHD of 19.6 to 21.6 mm when GHS was incorporating with a CCD. For comparison, we had no such length data in patients with GHS but without CCD. Third, this is a single case report. Our results cannot be safely extended to other patients in a similar condition.
Conclusion
Together with persisted CCD, a persisted GHS was correlated with a prolonged CCD, which was assumed to be one sign of motor deficits associated with the development of CCD. We concluded that CCD has a strong relationship with and exacerbates GHS. The AHD measurement of our patient with both CCD was greater than those of the general CCD-free stroke patients. Such larger AHD measurement was consistent with a more serious condition of CCD. Therefore, CCD could have worsened GHS. Our case also indicated the importance of the spino-reticulo-thalamic pathway, which goes upward ipsilaterally to the thalamus.
Acknowledgments
The authors would like to thank the patient who participated in this report.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Mon 23, Nov 2020Accepted: Wed 09, Dec 2020
Published: Thu 31, Dec 2020
Copyright
© 2023 Shin-Tsu Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GGR.2020.02.09
Author Info
Ting-Yu Tammy Hsieh Chuan-Ching Liu Hsin-Chen He Yuan-Yang Cheng Shin-Tsu Chang
Corresponding Author
Shin-Tsu ChangSchool of Medicine, National Defense Medical Center, Taipei, Taiwan
Figures & Tables

References
- Vuagnat H, Chantraine A (2003) Shoulder pain in hemiplegia revisited: Contribution of functional electrical stimulation and other therapies. J Rehabil Med 35: 49-54. [Crossref]
- Jeon S, Kim Y, Jung K, Chung Y (2017) The effects of electromyography-triggered electrical stimulation on shoulder subluxation, muscle activation, pain, and function in persons with stroke: A pilot study. NeuroRehabil 40: 69-75. [Crossref]
- Comley White N, Mudzi W, Musenge E (2018) Effects of shoulder strapping in patients with stroke: A randomised control trial. S Afr J Physiother 74: 430. [Crossref]
- Yang C, Chen P, Du W, Chen Q, Yang H et al. (2018) Musculoskeletal ultrasonography assessment of functional magnetic stimulation on the effect of glenohumeral subluxation in acute poststroke hemiplegic patients. BioMed Res Int 2018: 6085961. [Crossref]
- Karaahmet OZ, Gurcay E, Unal ZK, Cankurtaran D, Cakci A (2019) Effects of functional electrical stimulation-cycling on shoulder pain and subluxation in patients with acute-subacute stroke: a pilot study. Int J Rehabil Res 42: 36-40. [Crossref]
- Sommer WH, Bollwein C, Thierfelder KM, Baumann A, Janssen H et al. (2016) Crossed cerebellar diaschisis in patients with acute middle cerebral artery infarction: occurrence and perfusion characteristics. J Cereb Blood Flow Metab 36: 743-754. [Crossref]
- Infeld B, Davis SM, Lichtenstein M, Mitchell PJ, Hopper JL (1995) Crossed cerebellar diaschisis and brain recovery after stroke. Stroke 26: 90-95. [Crossref]
- Berker E, Smith A (1988) Diaschisis, site, time and other factors in raven performances of adults with focal cerebral lesions. Int J Neurosci 38: 267-285. [Crossref]
- Bohnen NI, Beran Koehn M, Mullan B, Fulgham JR (1998) Crossed cerebro-cellular diaschisis in a patients with melas with aphasia but without hemiparesis. Int J Neurosci 93: 181-184. [Crossref]
- Chang CC, Ku CH, Chang ST (2017) Postural asymmetry correlated with lateralization of cerebellar perfusion in persons with chronic stroke: A role of crossed cerebellar diaschisis in left side. Brain Inj 31: 90-97. [Crossref]
- He HC, Hsu MC, Hsu CS, Cheng YY, Chang ST (2018) Bidirectionality of the dentato-rubro-thalamo-cortical tract allows concurrent hypoperfusion in ipsilateral cerebellum and contralateral cerebral hemisphere: a case report. Medicine 97: e12590. [Crossref]
- Kim JS, Kim SH, Lim SH, Im S, Hong BY et al. (2019) Degeneration of the inferior cerebellar peduncle after middle cerebral artery stroke: another perspective on crossed cerebellar diaschisis. Stroke 50: 2700-2707. [Crossref]
- Hall J, Dudgeon B, Guthrie M (1995) Validity of clinical measures of shoulder subluxation in adults with poststroke hemiplegia. Am J Occup Ther 49: 526-533. [Crossref]
- Żyluk A, Puchalski P (2018) Effectiveness of complex regional pain syndrome treatment: A systematic review. Neurol Neurochir Pol 52: 326-333. [Crossref]
- Di Pietro F, McAuley JH, Parkitny L, Lotze M, Wand BM et al. (2013) Primary somatosensory cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J Pain 14: 1001-1018. [Crossref]
- Fukumoto M, Ushida T, Zinchuk VS, Yamamoto H, Yoshida S (1999) Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet 354: 1790-1791. [Crossref]
- Lai MH, Wang TY, Chang CC, Li TY, Chang ST (2008) Cerebellar diaschisis and contralateral thalamus hyperperfusion in a stroke patient with complex regional pain syndrome. J Clin Neurosci 15: 1166-1168. [Crossref]
- Serrati C, Marchal G, Rioux P, Viader F, Petit Taboué MC et al. (1994) Contralateral cerebellar hypometabolism: a predictor for stroke outcome? J Neurol Neurosurg Psychiatry 57: 174-179. [Crossref]
- Kim Y, Lim SH, Park GY (2019) Crossed cerebellar diaschisis has an adverse effect on functional outcome in the subacute rehabilitation phase of stroke: a case-control study. Arch Phys Med Rehabil 100: 1308-1316. [Crossref]
- Pertoldi S, Di Benedetto P (2005) Shoulder-hand syndrome after stroke. A complex regional pain syndrome. Eura Medicophys 41: 283-292. [Crossref]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM et al. (2011) Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 67: 942-968. [Crossref]
- Marenzana M, Chenu C (2008) Sympathetic nervous system and bone adaptive response to its mechanical environment. J Musculoskelet Neuronal Interact 8: 111-120. [Crossref]
- Watson JC, Sandroni P (2016) Central neuropathic pain syndromes. Mayo Clin Proc 91: 372-385. [Crossref]
- Umer M, Qadir I, Azam M (2012) Subacromial impingement syndrome. Orthop Rev 4: e18. [Crossref]
- Vanderstukken F, Maenhout A, Spanhove V, Jansen N, Mertens T et al. (2020) Quantifying acromiohumeral distance in elite male field hockey players compared to a non-athletic population. Braz J Phys Ther 24: 273-279. [Crossref]
- Yu DT, Friedman AS, Rosenfeld EL (2010) Electrical stimulation for treating chronic poststroke shoulder pain using a fully implanted microstimulator with internal battery. Am J Phys Med Rehabil 89: 423-428. [Crossref]
- Van Bladel A, Lambrecht G, Oostra KM, Vanderstraeten G, Cambier D (2017) A randomized controlled trial on the immediate and long-term effects of arm slings on shoulder subluxation in stroke patients. Eur J Phys Rehabil Med 53: 400-409. [Crossref]
- McCreesh KM, Crotty JM, Lewis JS (2015) Acromiohumeral distance measurement in rotator cuff tendinopathy: is there a reliable, clinically applicable method? A systematic review. Br J Sports Med 49: 298-305. [Crossref]
- Park GY, Kim JM, Sohn SI, Shin IH, Lee MY (2007) Ultrasonographic measurement of shoulder subluxation in patients with post-stroke hemiplegia. J Rehabil Med 39: 526-530. [Crossref]
