Perioperative Concerns For An Infant with Stuve-Wiedemann Syndrome: A Case Report
A B S T R A C T
Stuve-Wiedmann syndrome (SWS) is a rare autosomal recessive genetic disorder associated with skeletal abnormalities and dysautonomia, including hyperthermia. There are few reported anaesthetics of SWS patients. As a result, little is known about anaesthetic care for these patients. We report a case of SWS that was confirmed by identification of a disease-causing mutation in the LIFR gene. We discuss anaesthetic challenges associated with management of these patients, especially as it pertains to the historical association between SWS and malignant hyperthermia. We recommend a conservative approach to management of these patients given their propensity for fevers and the variable penetrance of the malignant hyperthermia phenotype when found in association with other genetic variations.
Keywords
Stuve-Wiedemann syndrome, anaesthesia, malignant hyperthermia, schwartz-jampel syndrome
Introduction
Stuve-Wiedemann syndrome (SWS) is a rare, autosomal recessive disorder first described in 1971 [1]. The prevalence of SWS may be as high as 5 in 100,000 births in some regions [2]. SWS results from loss of function mutations in the LIFR gene that codes for a transmembrane protein involved in IL-6 cytokine family signaling. Phenotypic features include skeletal abnormalities (bowed legs, joint restrictions, camptodactyly, and recurrent spontaneous fractures) and dysautonomia including otherwise unexplained and frequently lethal episodes of hyperthermia. The neonatal course is complicated by respiratory distress and feeding difficulties [2, 3].
Anaesthetic management of SWS patients presents many unanswered questions since SWS has been confused with phenotypically similar syndromes such as campomelic dysplasia, Crisponi syndrome, and Schwartz-Jampel syndrome (SJS) in the past. Without definitive genetic diagnoses, it is difficult to apply historical literature to current practice. SWS is now known to be the same syndrome as a subtype of SJS, SJS Type 2 [2, 3]. SWS is, however, genetically distinct from Schwartz-Jampel syndrome Type 1, Campomelic dysplasia, and Crisponi syndrome [4, 5]. Reports of anaesthetics with unequivocal genetic diagnosis of Stuve-Wiedemann Syndrome are lacking.
Case Report
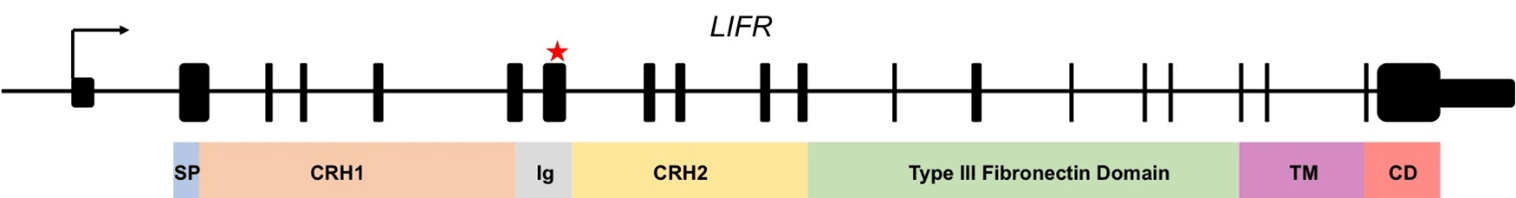
We present the anaesthetic of a 10-week-old infant undergoing gastrostomy tube placement. The patient was born to an otherwise healthy mother at term via Cesarean section. Notable findings at birth were small for gestational age, bilateral upper extremity contractures with clenched hands, and lower extremity bowing. Chest x-ray demonstrated bilateral pulmonary opacities. Skeletal imaging demonstrated widespread dysplasia with repeat studies demonstrating multiple long bone fractures. An echo demonstrated pulmonary hypertension with an estimated right ventricular systolic pressure of 73 mmHg. The neonatal course was complicated by persistent hemodynamic instability, pulmonary hypertension, and respiratory failure managed with inhaled nitric oxide, steroids, paralysis, and diuresis. Airway management during her ICU course included intubation on three separate occasions with each occasion involving more than one attempt. The patient’s course was notable for recurrent fevers with negative infectious work up. Whole exome-sequencing demonstrated a homozygous mutation in the LIFR gene that results in a frameshift mutation and premature stop codon in the translated protein (Figure 1).
On the day of surgery, the child was afebrile with a heart rate of 160 beats per minute and a blood pressure of 89/61 mmHg. She was breathing 48 breaths per minute and saturating 99% on 0.2 L nasal cannula. Her weight was 4.25 kg. Her airway exam was notable for micrognathia, clear secretions, nasal flaring, tracheal tugging, and subcostal retractions. Relevant preoperative medications included acetaminophen and clonidine for dysautonomia.
Figure 1: The LIFR gene consists of 20 exons. At the N-terminus there is a signal peptide (SP), followed by a cytokine receptor homology domain (CRH), an Ig-like domain, a second CRH domain, a type III fibronectin domain, a transmembrane domain (TM), and a cytoplasmic domain (CD). The patient presented herein was identified to have a previously described duplication in exon 7 that results in a frame shift mutation within the Ig-like domain of the protein. This leads to a truncated protein product. This mutation has previously been reported as disease-causing.
She was brought to the operating table with careful positioning given her multiple long bone fractures. Intramuscular ketamine and 50% nitrous oxide were used for initial sedation. Careful attempts at intravenous access were made given the frailty of her long bones and pre-existing fractures. Once intravenous access was obtained she was administered glycopyrrolate and induced with propofol and cisatracurium. Mask ventilation was successful with two hands, and the patient was intubated via a video laryngoscope on the first attempt. Maintenance was achieved with a propofol infusion. Her intraoperative course was notable for a transient temperature of 37.9ºC that self-resolved. She was extubated to 0.5 L nasal cannula and transferred to the intensive care unit. She remained on scheduled tylenol. On postoperative day 1, she had a temperature of 38.9ºC that resolved promptly without intervention. Her postoperative course was otherwise unremarkable.
Discussion
Management of SWS presents multiple challenges to the anaesthetist. In the case presented herein, skeletal abnormalities and fragility complicated obtaining intravenous access. In anticipation of a potentially difficult airway, and to minimize head manipulation, video laryngoscopy was used.
Foremost among the questions to be answered when caring for a SWS patient is whether or not a non-triggering anaesthetic should be employed. In 1973, two cases of SJS were reported and one of these patients developed hyperthermia under nitrous/halothane anaesthesia [6]. Subsequently, a report described a patient with SJS who received atropine, nitrous, curare, and ketamine for repair of a cleft palate and then developed hyperthermia, tachycardia, and elevation of blood pressure resulting in cancellation of the procedure [7]. These cases predate the differentiation of SJS into Type 1a, Type 1b, and Type 2 (which is synonymous with SWS). It is therefore unclear whether or not these patients had SWS [8].
More recently, anaesthetics of a single patient with genetically-confirmed SWS were reported. This patient underwent six separate anaesthetics all using volatile agents. In one instance, he had postoperative hyperthermia to 39ºC resolving with paracetamol. Given the patient’s repeated tolerance of sevoflurane, the authors concluded that triggering anaesthetics such as sevoflurane are safe in SWS [4]. While this may provide some reassurance, it should be noted that even in patients with ryanodine receptor mutations known to cause malignant hyperthermia, the penetrance of malignant hyperthermia is variable. Indeed, some such patients undergo multiple anaesthetics without issue only to present with malignant hyperthermia at a later date [9].
In our case, we chose to pursue a conservative approach to management by avoiding a triggering anaesthetic. We hypothesized that the risk of malignant hyperthermia was low given the distinct genetic cause of SWS relative to known causes of malignant hyperthermia (RYR1, CACNA1S, and STAC3 mutations) [9]. Nonetheless, our patient had persistent fevers and dysautonomia throughout her hospital course. These fevers are known to be life threatening in SWS. We therefore decided it prudent to avoid diagnostic uncertainty should a peri-operative fever arise by avoiding triggering agents altogether. Indeed, our patient’s brief intra- and post-operative fevers despite a non-triggering anaesthetic illustrates the practicality of this approach.
Management of children with rare syndromes can prove challenging given the paucity of data surrounding their anaesthetic management. While a previous report based on a single patient challenged the association between SWS and malignant hyperthermia, we pursued a conservative approach similar to the recently reported anaesthetic for an adult case of SWS with the rational that this may simplify evaluation of perioperative fever that is likely to be common in these patients [4, 10]. We also emphasize that even in the classical case of RYR1 gene mutations there is a variable relationship between genotype and phenotype that is likely influenced by other genetic, epigenetic, and environmental factors. Therefore, uneventful delivery of triggering agents in a small number of cases cannot widely guarantee their safety.
Funding
None.
Conflicts of Interest
None.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data
Not applicable.
Author Contributions
Christopher J Mariani: writing and editing of manuscript. Gennadiy Fuzaylov: writing and editing of manuscript.
Article Info
Article Type
Case ReportPublication history
Received: Wed 18, Nov 2020Accepted: Fri 04, Dec 2020
Published: Mon 21, Dec 2020
Copyright
© 2023 Christopher J. Mariani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.12.14
Author Info
Christopher J. Mariani Gennadiy Fuzaylov
Corresponding Author
Christopher J. MarianiDepartment of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA
Figures & Tables
References
- Stuve A, Wiedemann HR (1971) Congenital bowing of the long bones in two sisters. Lancet 2: 495. [Crossref]
- Mikelonis D, Jorcyk CL, Tawara K, Oxford JT (2014) Stuve-Wiedemann syndrome: LIFR and associated cytokines in clinical course and etiology. Orphanet J Rare Dis 9: 34. [Crossref]
- Dagoneau N, Scheffer D, Huber C, Al Gazali LI, Rocco MD et al. (2004) Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am J Hum Genet 74: 298-305. [Crossref]
- Bonthuis D, Morava E, Booij LH, Driessen JJ (2009) Stuve Wiedemann syndrome and related syndromes: case report and possible anesthetic complications. Paediatr Anaesth 19: 212-217. [Crossref]
- Wiedemann HR, Stuve A (1996) Stuve-Wiedemann syndrome: update and historical footnote. Am J Med Genet 63: 12-16. [Crossref]
- Fowler WM Jr., Layzer RB, Taylor RG, Eberle ED, Sims GE et al. (1974) The Schwartz-Jampel syndrome. Its clinical, physiological and histological expressions. J Neurol Sci 22: 127-146. [Crossref]
- Seay AR, Ziter FA (1978) Malignant hyperpyrexia in a patient with Schwartz-Jampel syndrome. J Pediatr 93: 83-84. [Crossref]
- Giedion A, Boltshauser E, Briner J, Eich G, Exner G et al. (1997) Heterogeneity in Schwartz-Jampel chondrodystrophic myotonia. Eur J Pediatr 156: 214-223. [Crossref]
- Ibarra Moreno CA, Hu S, Kraeva N, Schuster F, Johannse S et al. (2019) An Assessment of Penetrance and Clinical Expression of Malignant Hyperthermia in Individuals Carrying Diagnostic Ryanodine Receptor 1 Gene Mutations. Anesthesiology 131: 983-991. [Crossref]
- Artilheiro V, Portela F, Reis AT (2020) Anesthesia for Stuve-Wiedemann syndrome: a rare adult patient case report. J Appl Genet. 61: 571-573. [Crossref]
