Patient-Related Factors Affecting the Sensitivity of Sonication of Removed Hip and Knee Implants Against Conventional Tissue Cultures for Diagnosis of Prosthetic Joint Infections
A B S T R A C T
Despite its decreasing incidence, prosthesis-related infections remain a research, diagnostic, therapeutic and cost-related problem. Our study aim was to compare the diagnostic accuracy of conventional periprosthetic tissue culture and culture of sonication fluid of the explanted hardware and to investigate the role of patient- related factors affecting the sensitivity of the sonication method. We investigated 70 patients undergoing revision hip or knee arthroplasty, at our institution. Patients’ medical history and demographic characteristics were recorded. We compared the culture of samples obtained by sonication of explanted hip and knee prostheses with conventional culture of periprosthetic tissue for the microbiological diagnosis of prosthetic-joint infection. Thirty-two patients had septic loosening and 38 aseptic loosening (48 hip prostheses and 22 knee prostheses). The sensitivities of sonication fluid culture and conventional tissue cultures were 81.25% and 56.25%, respectively (p-value = 0.043). The sensitivity of the sonication method was statistically higher in obese, diabetic patients, with age above 60, in uncemented arthroplasties and in arthroplasties because of primary osteoarthritis (p-values < 0.05). The sonication method has a greater sensitivity than the conventional periprosthetic tissue cultures for the periprosthetic infections, especially in obese, diabetic patients, with age above 60, in uncemented arthroplasties and in arthroplasties because of primary osteoarthritis.
Keywords
Sonication, biofilm, orthopedic implants, total hip and knee arthroplasty infections
Introduction
Total hip and knee replacements are two of the most common orthopedic surgical procedures. Periprosthetic joint infection (PJI) is the most challenging complication associated with total joint arthroplasty [1]. Despite considerable progress in prevention and treatment of PJIs, the absolute number of patients with such infections is rising due to the lifelong risk for bacterial seeding of the implant [2]. PJIs occur less frequently than aseptic failures but represent the most devastating complication with high morbidity and substantial cost.
In patients with primary knee replacement, the infection rate has been reported to be 0,8 to 3,3%, and in those with hip replacement is 0,3% to 3,0%. Infection rates after revision surgery are usually considerably higher (5-40%) [3, 4]. In the future, it is expected that the incidence of PJIs will further increase due to more sophisticated detection methods for the microbial biofilms, the growing number of implanted prostheses in the aging population and the increasing residency time of prostheses [5]. PJI is considered to be associated with the presence of bacterial biofilms attached to the implant, where the bacteria have changed their phenotypes to an extremely resistant form of life [6]. Conventionally, the microbiological diagnosis of PJI has been based on periprosthetic tissue cultures (PTC). However, the sensitivity of conventional culture methods does not exceed 75% [7]. Because of previous use of antibiotics, sampling errors, inadequate quantities of vital bacteria retrieved, inappropriate transport or fastidious organisms, more than 20% of PJIs result in being culture negative [8, 9]. Another reason for negative cultures is the adherence of bacteria within the biofilm which may inhibit the detection of the pathogens that cause PJIs [6, 10]. Thus, disrupting the biofilm from the surface of the explanted prostheses to free the bacteria can improve the results of the culture and the outcome of the treatment of the infection.
The application of long-wave ultrasound before cultures for the disruption of the prostheses biofilm and the enhancement of the bacterial growth has been first described by Trampuz et al in 2007 [11]. In their study, they reported that sonication before culture of the explanted hip or knee implants for the dislodgement of the adherent bacteria yielded a significant better recovery of bacterial growth in culture than the conventional culture of periprosthetic tissue samples for the microbiological diagnosis of PJIs.
The purpose of this study was to compare the diagnostic accuracy of conventional periprosthetic tissue culture and culture of fluid derived from vortexing and bath sonication of the explanted total hip and knee prostheses. We performed a descriptive data analysis comparing the detection of pathogens in the sonication fluid culture (SFC) methods versus the conventional-culture methods of the periprosthetic tissue. Possible metabolic factors from patients’ history, such as hypertension, diabetes mellitus, lipid abnormality, hypertriglyceridemia, bone mass index (BMI) and metabolic syndrome (MS) that could probably affect the sensitivity of the SFC, along with reason for arthroplasty, type and site of infection and use of cement were also investigated.
Materials & Methods
Between October 2011 and November 2013, we investigated 70 patients, 28 men and 42 women, undergoing revision hip or knee arthroplasty because of loosening of the prostheses, at our hospital. Forty eight patients had undergone total hip replacement and 22 total knee replacements. The explanted hardware were separated in sterile containers and sonicated under sterile conditions. If patients showed preoperative signs of infections, a synovial aspiration was performed, preoperatively. Demographic characteristics, clinical, laboratory and microbiological data of the patients were recorded.
I Definition of PJI
For the definition of PJI we used the Infectious Diseases Society of America (IDSA) Guidelines [12]. According to these guidelines, one of the following criteria is definitive evidence of PJI: a) presence of sinus tract that communicates with the prosthesis, b) presence of acute inflammation on the histopathologic examination of the periprosthetic tissue, c) presence of visible purulence surrounding the prosthesis and d) two or more positive intraoperative PTCs or positive SFC. A sonication fluid culture was considered positive when it yielded > 50 colony – forming units (CFU)/ml of the same organism. Isolation of a bacterial species in a single tissue sample or yielding < 50 CFU/ml in sonication fluid were classified as false positive. Aseptic failure was defined as loosening of the prosthesis in absence of any of these criteria. PJIs were classified according to the onset of symptoms, as early, delayed and late (less than 3 months, 3 – 24 months, more than 24 months, respectively) [13]. Inflammation in the histopathological examination was defined as 5 neutrophils per high-power field. Previous antimicrobial therapy was defined as administration of antimicrobial agents during the 14 days before removal of the prostheses.
II Definition of Metabolic Syndrome
According to the US National Cholesterol Education Program Adult Treatment Panel III (2001), metabolic syndrome was defined by the presence of at least 3 of the following 5 factors: a) Hypertension (defined as blood pressure ≥ 130/85 mmHg or treatment with anti-hypertensive drugs) b) Diabetes mellitus (defined as fasting plasma glucose ≥6.1 mmol/L or treatment with anti-diabetic drugs) c) Hypertriglyceridemia (defined as triglycerides > 150 mg/dl or appropriate treatment) d) Dyslipidemia (defined as HDL-C < 40 mg/dL in males, < 50 mg/dL in females or appropriate treatment e) Central obesity (defined as waist circumference ≥ 102 cm in males and ≥ 88 cm in females) [14].
III Periprosthetic Tissue Cultures
For all patients, at least five intraoperative periprosthetic tissue specimens were collected from the bone-cement/bone-prosthesis interface, from capsule and from soft tissues with obvious inflammatory changes. Tissue specimens were located into sterile boxes and individually homogenized in 3 ml Trypticase Soy Broth for 1 min using mortar and pestle. Tissue homogenate samples were inoculated in 0.1 ml aliquots onto aerobic (SBA) and anaerobic sheep blood agar (ASBA) plates and in 1 mL aliquots into thioglycolate broth. The cultures were incubated at 37οC for 10 days. A terminal subculture was performed from all thioglycolate broth specimens on blood agar plates and incubated at 37οC for 5 more days. Positive tissue cultures were considered those with the same microorganism isolation of at least two periprosthetic tissue samples. Each unique colony of isolated microorganisms was identified, and their antimicrobial susceptibility was tested by using standard automatic methods (Vitek-2 system; bio Mérieux, Marcy L’Etoile, France). Two more periprosthetic tissue samples were sent for histological examination.
IV Sonication Fluid Cultures
The explanted prosthesis (or its components) was aseptically removed in the operating room and located in a widemouthed, sterile, solid air-tight container (Lock & Lock; Vertrag AG, Stafa, Switzerland). The implant was transported to the microbiology laboratory and sonicated within 6 h. Sonication of the implant was performed according to the Trampuz et al. method [11].
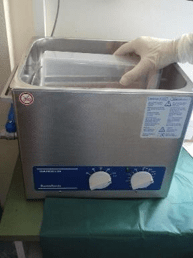
Briefly, sterile Ringer solution (solution volume ranged from 50 to 400 ml depending on the size of implant) was added to the container in a laminar airflow biosafety cabinet to cover 85–90% of the volume of a big sized prosthesis or the entire volume of small sized components. The container with the implant was vortexed for 30 sec, followed by sonication for 1 min (at a frequency of 40 kHz and power density of 0.22 W/cm2), as determined by a calibrated hydrophone (type 8103; Bruel and Kjær, Naerum, Denmark). As shown in (Figure 1), for sonication, ultrasound bath BactoSonic (Bandelin GmbH, Berlin, Germany) was used according to the manufacturer’s instructions.
Figure 1: Ultrasound bath.
No differences in frequency or power density were observed at various locations within the ultrasound bath during the study period. The container was subsequently vortexed for an additional 30 sec to remove any residual microorganisms and to homogeneously distribute them in the sonication fluid. Aliquots of 0.1 ml sonicate fluid were inoculated onto sheep blood agar (SBA) and anaerobic sheep blood agar (ASBA) plates. Additionally, 1 ml of the remaining of sonication fluid was added in 10 ml thioglycollate broth (TGB). The SBA plates and TSB were incubated at 37οC aerobically and the ASBA plates and TGB at 37οC anaerobically and inspected daily for bacterial growth. The criterium used to interpret sonicate fluid culture positivity was a cutoff value of at least 50 CFU/mL sonication fluid Every distinct morphotype colony of microorganisms on plates was enumerated (i.e., number of CFU/mL sonication fluid) and its identification and susceptibility testing was performed by using standard automatic methods (Vitek-2 system; bio Mérieux, Marcy L’Etoile, France).
V Statistical Analysis
Student’s unpaired t test was used to compare continuous values, such as age, BMI among cases and controls and among any other subgroups. Categorical variables were compared using the Fischer’s exact test. The sensitivity of the different culture methods was compared by McNemar’s test of paired proportions. The sensitivities, specificities, positive predictive values and negative predictive values of the different methods were calculated with two-by-two contingency tables. Ninety-five percent confidence intervals (95% CI) were calculated as exact binomial confidence intervals. A probability p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using the PASW 18 (SPSS release 18.0; SPSS Inc., Chicago, Illinois).
Results
Of the 70 patients who were operated because of loosening of total hip and knee arthroplasty, 32 patients had septic loosening and 38 aseptic loosening (48 hip prostheses and 22 knee prostheses). Demographic, clinical characteristics and type of surgery are shown in (Table 1). The mean age of the patients was 69.2 ± 10.86 years (range 47 – 89 years). Primary osteoarthritis was the most common cause for both total hip and knee arthroplasty. The median time from implantation to revision or resection surgery was longer in patients with aseptic loosening of total knee replacement than in patients with periprosthetic knee infection (45 months vs 8 months, respectively, p-value = 0.007). There were 4 early PJIs, 12 delayed PJIs and 16 late PJIs.
No patient had received any antibiotic therapy for 14 days prior to revision or resection surgery. Table 2 demonstrates the sensitivity of the culture of sonication fluid versus the culture of periprosthetic tissue in different study groups. The sensitivity of SFC was 81.25% (95%CI: 0.62-0.93) and the sensitivity of PTC was 56.25% (95%CI: 0.37-0.73, p–value = 0.043). Specificity was 94.74% (95%CI: 0.82-0.99) for both methods. Sensitivity of SFC was higher than PTC in all patient subcategories, but its superiority was statistically established in patients older than 60 years, in obese patients, in diabetic patients and in uncemented arthroplasties. The sensitivity of the histopathological examination of the periprosthetic tissue was 68.75% (95%CI: 0.50- 0.84). There were 10 patients where the isolated pathogen was detected in SFC but not in PTC, while in 2 cases the pathogen was detected only in PTC. There were 4 patients where no bacteria were detected by any microbiological method and the diagnosis was based on clinical and histological findings, according to IDSA guidelines. In cases of infection, coagulase-negative staphylococci were isolated in 43.8% of patients; staphylococcus aureus was isolated in 12.5% of patients, Gram negative bacteria in 37.5% of patients, and other bacteria in 6.2% of patients. As shown in (Table 1), among patients with PJI, 9 patients underwent 1-stage exchange, 22 patients had 2-stage exchange and one patient was subjected to knee arthrodesis. For patients who had 2-stage exchange, the median time to reimplantation was 66.25 days.
Discussion
In prosthetic joint infections, organisms attached to the prosthesis often form protective biofilms which make them resistant to antibiotics and difficult to detect with conventional tissue cultures [11, 15]. Sonication dislodges these bacteria from the prosthesis allowing them to be cultured [11]. Understanding the etiology of infection is very important, as it allows selection of the best antimicrobial therapy. The results of our study demonstrate that in our group of hip and knee PJIs, SFC had an overall sensitivity of 81.25% in comparison to 56.25% sensitivity of PTC. This difference was found to be statistically significant (p-value = 0.043). Both investigations had the same specificity of 94.74%. In agreement is the study by Trampuz et al with similar numbers of reported sensitivity for SFC (78.5%) and PTC (60.8%) [11]. Furthermore, the higher sensitivity of sonication method as opposed to tissue cultures has been confirmed by many previous studies and recent meta-analyses [16- 22].
Biofilms are easier to form in cases where host immunity is lowered such as obesity and diabetes and in elder patients [23]. Obesity is regarded as an inflammatory disease and has been correlated with an increased risk of infections [24]. It has been stipulated that circulated adipokines in obese patients negatively influence their immune response to microbial colonization [25-27] . A clinical study has demonstrated higher bacterial count in biofilms of the oral cavity in obese adolescents [27]. As a result, PTCs will have a lower sensitivity in these patients. On the contrary, sonication dislodges bacteria from the biofilm and allows the sonication fluid to produce positive cultures. In our study, SFC had a statistically significant higher sensitivity (85.7%) in obese patients (BMI > 30) as opposed to PTC (42.85%, p-value = 0.041). Respectively, the sensitivity of sonication method was higher in people older than 60 (75% versus 41.7%, p-value = 0.041). Our findings can be explained by the aforementioned impairment of the immune system in elder and obese patients.
Table 1: Characteristics of study subjects.
|
Characteristics |
Subjects with Aseptic Loosening (n = 38) |
Subjects with PJI (n = 32) |
P-value |
|
Mean age (years) |
69.68 ± 9,31 (range 50 – 83) |
68.25 ± 10.86 (range 47 – 89) |
0.662 |
|
Sex |
|
|
0.808 |
|
Male |
16 (42%) |
12 (38%) |
|
|
Female |
22 (58%) |
20 (62%) |
|
|
Reason for arthroplasty |
|
|
|
|
Primary Osteoarthritis |
24 (63%) |
18 (56%) |
0.628 |
|
Inflammatory joint disorder |
4 (10%) |
0 |
0.119 |
|
Fracture / Trauma |
7 (18%) |
12 (38%) |
0.268 |
|
Congenital abnormalities |
2 (5%) |
2 (6%) |
1.000 |
|
Other |
1 (3%) |
0 |
1.000 |
|
Associated conditions |
|
|
|
|
Hypertension |
24 (63%) |
18 (56%) |
0.628 |
|
Diabetes mellitus |
10 (31%) |
11 (34%) |
0.601 |
|
Lipid abnormality |
9 (24%) |
7 (22%) |
1.000 |
|
Hypertriglyceridemia |
1 (3%) |
2 (6%) |
0.589 |
|
BMI |
28,74 ± 7,42 (range 20,76 – 47,75) |
28,89 ± 3,73 (range 22,83 – 34,77) |
0.921 |
|
Metabolic Syndrome |
9 (24%) |
7 (22%) |
1.000 |
|
Site of arthroplasty |
|
|
0.616 |
|
Hip |
25 (66%) |
23 (72%) |
|
|
Knee |
13 (34%) |
9 (28%) |
|
|
Cemented arthroplasties |
|
|
0.610 |
|
Cemented |
11 (29%) |
12 (38%) |
|
|
Uncemented |
27 (71%) |
20 (62%) |
|
|
Age of implant (months) |
105.72 ± 119.73 (range 1 – 336) |
74.94 ± 126.29 (range 2 – 432) |
0.306 |
|
Hip |
136.00 ± 135.02 |
105.18 ± 143.08 |
0.456 |
|
Knee |
45.17 ± 38.68 |
8.40 ± 3.57 |
0.007 |
|
PJI components |
|
|
|
|
Sinus tract |
0 |
7 |
0.003 |
|
Visible purulence |
0 |
15 |
<0.001 |
|
Positive cultures |
0 |
18 |
<0.001 |
|
Tissue inflammation |
0 |
21 |
<0.001 |
|
Surgical procedure |
|
|
|
|
One-stage exchange |
34 (89%) |
9 (28%) |
|
|
Two-stage exchange |
4 (11%) |
22 (69%) |
|
|
Arthrodesis |
|
1 (3%) |
|
In our study, SFC had a statistically significant higher sensitivity (100%) in diabetic patients as opposed to PTC (36.4%, p-value = 0.041). Our findings can be explained by the fact that diabetes related infections frequently form polymicrobial antibiotic resistant biofilms, demonstrated by many studies [28-30] . A further in vivo study by Watters et al showed that diabetic mice were prone to develop biofilms and subsequent problems with wound healing [31].
According to the results of our study, SFC demonstrated a better sensitivity of 87.5% in patients with late infections as opposed to 62.5% of PTC. In agreement is a recent study of 317 PJIs where SFC was found to have higher diagnostic accuracy in patients with late infections. The theory behind this is that in acute infections the micro-organisms have not formed biofilms as yet and therefore tissue culture has also good sensitivity [15]. Nevertheless, in our study, SFC demonstrated higher sensitivity in early infections as well (100%) as opposed to PTCs (50%). However, these differences in sensitivity are not statistically significant.
Our results demonstrated that SFC was more sensitive in PJIs around uncemented prostheses (80% versus 50%, p-value = 0.041). We stipulate that the reason behind this is that the antibiotic loaded cement used in cemented arthroplasties prevents to a certain extent the formation of a biofilm. It is of note though that an in vitro study by Kendall et al demonstrated that bacteria can adhere and grow on antibiotic-loaded cement [32] .
Table 2: Sensitivity of the culture of the sonication fluid versus the culture of periprosthetic tissue.
|
|
Sensitivity of sonication fluid culture |
Sensitivity of periprosthetic tissue culture |
P - value |
|
Overall |
81.25% |
56.25% |
0.043 |
|
Type of infection |
|
|
|
|
Early |
100% |
50% |
0.479 |
|
Delayed |
66.67% |
50% |
0.479 |
|
Late |
87.5% |
62.5% |
0.288 |
|
Site of infection |
|
|
|
|
Hip |
90.91% |
63.63% |
0.114 |
|
Knee |
66.67% |
55.55% |
0.479 |
|
Age |
|
|
|
|
< 60 |
100% |
100% |
1.000 |
|
> 60 |
75% |
41.67% |
0.043 |
|
Gender |
|
|
|
|
Male |
83.33% |
50% |
0.134 |
|
Female |
80% |
60% |
0.289 |
|
Hypertension |
|
|
|
|
Yes |
66.67% |
33.33% |
0.114 |
|
No |
100% |
85.71% |
0.479 |
|
Diabetes mellitus |
|
|
|
|
Yes |
100% |
36.37% |
0.041 |
|
No |
76.19% |
66.67% |
0.683 |
|
Dyslipidemia |
|
|
|
|
Yes |
57.14% |
57.14% |
1.000 |
|
No |
88% |
56% |
0.114 |
|
Metabolic syndrome |
|
|
|
|
Yes |
100% |
42.86% |
0.137 |
|
No |
72% |
56% |
0.289 |
|
BMI |
|
|
|
|
< 25 |
75% |
75% |
1.000 |
|
> 25 |
83.33% |
50% |
0.133 |
|
25 – 30 |
80% |
60% |
0.479 |
|
> 30 |
85.71% |
42.85% |
0.041 |
|
Reason for arthroplasty |
|
|
|
|
Primary OA |
77.78% |
44.44% |
0.041 |
|
Trauma |
83.33% |
83.33% |
1.000 |
|
Use of cement |
|
|
|
|
Cemented arthroplasties |
83.33% |
66.67% |
0.683 |
|
Uncemented arthroplasties |
80% |
50% |
0.041 |
Culture of retrieved gentamicin-loaded bead and antibiotic-loaded cement spacers following second stage revision for PJIs has also shown that micro-organisms can grow on these surfaces [33, 34] . Our finding that the sonication method was more sensitive than PTC in arthroplasties because of primary osteoarthritis (77.8% versus 44.4%, p-value = 0.041) can be explained by the diversity and adaptability in biofilm communities caused by increased endogenous oxidative stress, a condition that is a main characteristic of osteoarthritis [35].
Our study represents a prospective study of patients with PJIs comparing the results of sonication with standard tissue cultures. To the best of our knowledge, this is the first study in the literature to have produced results of SFC in relation to patients’ age and comorbidities such as BMI and diabetes and also according to the type of the prosthesis and the cause of arthroplasty. The influence of other factors, such as site of infection, gender, hypertension, dyslipidemia and metabolic syndrome in the sensitivity of sonication method is existent but not statistically significant. Limitations of our study include the relatively small number of patients as well as that only hip and knee arthroplasties were included. Furthermore, none of the patients received antibiotics within 2 weeks from surgery which did not give us the opportunity to assess the effect of this parameter in the comparison between sonicate fluid and tissue cultures.
In 2013 the International Consensus Meeting on Periprosthetic Joint infection advocated against the routine sonication of explanted prosthesis. The group concluded that SFC should be used in cases of suspected or proven prosthetic joint infections in which preoperative aspirates have failed to reveal any pathogens and in cases where antibiotics have been administered within 2 weeks from revision surgery [36]. The study by Puig-Verdie et al declared that the SFC is recommended only in delayed implant failures [15]. On the other hand, other published studies have come to conclusion that the sonication method is quite reliable and sufficient for pathogen detection in the clinical diagnostic routine [37-39]. We believe that the use of sonication is a cheap and useful test, with high sensitivity in all patient subcategories and should be applied as a routine in PJI investigation.
The results of our study demonstrate that SFC is statistically significant more sensitive than PTC for PJIs around knee and hip arthroplasties. Furthermore, the SFC has been statistically significant more sensitive in diabetics, obese patients, patients > 60 years old, in uncemented prostheses and in arthroplasties because of primary osteoarthritis. Taking into consideration that in patients with PJIs the tissue cultures are frequently negative, we advocate the broad use of sonication. Especially in cases of suspected PJIs in elder people with diabetes and obesity we strongly advise for the routine use of sonication in order to reach the correct diagnosis and apply the appropriate treatment for these sensitive groups of patients. Our findings need to be confirmed in a larger patient series and in other joint arthroplasties.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 19, Dec 2019Accepted: Sat 04, Jan 2020
Published: Wed 15, Jan 2020
Copyright
© 2023 Panagiotis Lepetsos. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.CMR.2019.01.04
Author Info
Anastasios Gketsos Antonios Kardatos Antonios Stylianakis Ioannis Giannaris Nikolaos Baxevanos Nikolaos Liarakos Panagiotis Lepetsos
Corresponding Author
Panagiotis LepetsosKAT Hospital, Nikis 2, 14561, Kifissia, Athens, Greece
Figures & Tables
Table 1: Characteristics of study subjects.
|
Characteristics |
Subjects with Aseptic Loosening (n = 38) |
Subjects with PJI (n = 32) |
P-value |
|
Mean age (years) |
69.68 ± 9,31 (range 50 – 83) |
68.25 ± 10.86 (range 47 – 89) |
0.662 |
|
Sex |
|
|
0.808 |
|
Male |
16 (42%) |
12 (38%) |
|
|
Female |
22 (58%) |
20 (62%) |
|
|
Reason for arthroplasty |
|
|
|
|
Primary Osteoarthritis |
24 (63%) |
18 (56%) |
0.628 |
|
Inflammatory joint disorder |
4 (10%) |
0 |
0.119 |
|
Fracture / Trauma |
7 (18%) |
12 (38%) |
0.268 |
|
Congenital abnormalities |
2 (5%) |
2 (6%) |
1.000 |
|
Other |
1 (3%) |
0 |
1.000 |
|
Associated conditions |
|
|
|
|
Hypertension |
24 (63%) |
18 (56%) |
0.628 |
|
Diabetes mellitus |
10 (31%) |
11 (34%) |
0.601 |
|
Lipid abnormality |
9 (24%) |
7 (22%) |
1.000 |
|
Hypertriglyceridemia |
1 (3%) |
2 (6%) |
0.589 |
|
BMI |
28,74 ± 7,42 (range 20,76 – 47,75) |
28,89 ± 3,73 (range 22,83 – 34,77) |
0.921 |
|
Metabolic Syndrome |
9 (24%) |
7 (22%) |
1.000 |
|
Site of arthroplasty |
|
|
0.616 |
|
Hip |
25 (66%) |
23 (72%) |
|
|
Knee |
13 (34%) |
9 (28%) |
|
|
Cemented arthroplasties |
|
|
0.610 |
|
Cemented |
11 (29%) |
12 (38%) |
|
|
Uncemented |
27 (71%) |
20 (62%) |
|
|
Age of implant (months) |
105.72 ± 119.73 (range 1 – 336) |
74.94 ± 126.29 (range 2 – 432) |
0.306 |
|
Hip |
136.00 ± 135.02 |
105.18 ± 143.08 |
0.456 |
|
Knee |
45.17 ± 38.68 |
8.40 ± 3.57 |
0.007 |
|
PJI components |
|
|
|
|
Sinus tract |
0 |
7 |
0.003 |
|
Visible purulence |
0 |
15 |
<0.001 |
|
Positive cultures |
0 |
18 |
<0.001 |
|
Tissue inflammation |
0 |
21 |
<0.001 |
|
Surgical procedure |
|
|
|
|
One-stage exchange |
34 (89%) |
9 (28%) |
|
|
Two-stage exchange |
4 (11%) |
22 (69%) |
|
|
Arthrodesis |
|
1 (3%) |
|
Table 2: Sensitivity of the culture of the sonication fluid versus the culture of periprosthetic tissue.
|
|
Sensitivity of sonication fluid culture |
Sensitivity of periprosthetic tissue culture |
P - value |
|
Overall |
81.25% |
56.25% |
0.043 |
|
Type of infection |
|
|
|
|
Early |
100% |
50% |
0.479 |
|
Delayed |
66.67% |
50% |
0.479 |
|
Late |
87.5% |
62.5% |
0.288 |
|
Site of infection |
|
|
|
|
Hip |
90.91% |
63.63% |
0.114 |
|
Knee |
66.67% |
55.55% |
0.479 |
|
Age |
|
|
|
|
< 60 |
100% |
100% |
1.000 |
|
> 60 |
75% |
41.67% |
0.043 |
|
Gender |
|
|
|
|
Male |
83.33% |
50% |
0.134 |
|
Female |
80% |
60% |
0.289 |
|
Hypertension |
|
|
|
|
Yes |
66.67% |
33.33% |
0.114 |
|
No |
100% |
85.71% |
0.479 |
|
Diabetes mellitus |
|
|
|
|
Yes |
100% |
36.37% |
0.041 |
|
No |
76.19% |
66.67% |
0.683 |
|
Dyslipidemia |
|
|
|
|
Yes |
57.14% |
57.14% |
1.000 |
|
No |
88% |
56% |
0.114 |
|
Metabolic syndrome |
|
|
|
|
Yes |
100% |
42.86% |
0.137 |
|
No |
72% |
56% |
0.289 |
|
BMI |
|
|
|
|
< 25 |
75% |
75% |
1.000 |
|
> 25 |
83.33% |
50% |
0.133 |
|
25 – 30 |
80% |
60% |
0.479 |
|
> 30 |
85.71% |
42.85% |
0.041 |
|
Reason for arthroplasty |
|
|
|
|
Primary OA |
77.78% |
44.44% |
0.041 |
|
Trauma |
83.33% |
83.33% |
1.000 |
|
Use of cement |
|
|
|
|
Cemented arthroplasties |
83.33% |
66.67% |
0.683 |
|
Uncemented arthroplasties |
80% |
50% |
0.041 |
References
- Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V et al. (2010) The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 468: 45-51. [Crossref]
- Zimmerli W, Trampuz A, , Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351: 1645-1654. [Crossref]
- Dale H, Fenstad AM, Hallan G, Havelin LI, Furnes O et al. (2012) Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 83: 449-458. [Crossref]
- Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J (2012) Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27: 61-65 e1. [Crossref]
- Campoccia D, Montanaro L, , Arciola CR (2006) The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27: 2331-2339. [Crossref]
- Costerton JW, Montanaro L, , Arciola CR (2005) Biofilm in implant infections: its production and regulation. Int J Artif Organs 28: 1062-1068. [Crossref]
- Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD et al. (2007) Culture-negative prosthetic joint infection. Clin Infect Dis 45: 1113-1119. [Crossref]
- Padgett DE, Silverman A, Sachjowicz F, Simpson RB, Rosenberg AG et al. (1995) Efficacy of intraoperative cultures obtained during revision total hip arthroplasty. J Arthroplasty 10: 420-426. [Crossref]
- Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM , Patel R (2003) Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthop Relat Res (414): 69-88. [Crossref]
- Buret A, Ward KH, Olson ME , Costerton JW (1991) An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Biomed Mater Res 25: 865-874. [Crossref]
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK et al. (2007) Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357: 654-663. [Crossref]
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W et al. (2013) Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of america. Clin Infect Dis 56: e1-e25. [Crossref]
- Zimmerli W, Ochsner PE (2003) Management of infection associated with prosthetic joints. Infection 31: 99-108. [Crossref]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) (2001). JAMA 285: 2486-2497. [Crossref]
- Puig-Verdié L, Alentorn-Geli E, González-Cuevas A, Sorlí L, Salvadó M et al. (2013) Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J 95-B: 244-249. [Crossref]
- Esteban J, Gomez-Barrena E, Cordero J, Martin-de-Hijas NZ, Kinnari TJ et al. (2008) Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol 46: 488-492. [Crossref]
- Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S et al. (2013) Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 37: 931-936. [Crossref]
- Portillo ME, Salvado M, Alier A, Martinez S, Sorli L et al. (2014) Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect 69: 35-41. [Crossref]
- Vergidis P, Greenwood-Quaintance KE, Sanchez-Sotelo J, Morrey BF, Steinmann SP et al. (2011) Implant sonication for the diagnosis of prosthetic elbow infection. J Shoulder Elbow Surg 20: 1275-1281. [Crossref]
- Janz V, Wassilew GI, Kribus M, Trampuz A, Perka C (2015) Improved identification of polymicrobial infection in total knee arthroplasty through sonicate fluid cultures. Arch Orthop Trauma Surg 135: 1453-1457. [Crossref]
- Liu H, Zhang Y, Li L, Zou HC (2016) The application of sonication in diagnosis of periprosthetic joint infection. Eur J Clin Microbiol Infect Dis 36: 1-9.[Crossref]
- Zhai Z, Li H, Qin A, Liu G, Liu X et al. (2014) Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J Clin Microbiol 52: 1730-1736. [Crossref]
- Hall-Stoodley L , Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11: 1034-1043. [Crossref]
- Das UN (2001) Is obesity an inflammatory condition? Nutrition 17: 953-966. [Crossref]
- Ahima, RS , Osei SY (2008) Adipokines in obesity. Front Horm Res 36: 182-197. [Crossref]
- Huttunen, R , Syrjanen J (2013) Obesity and the risk and outcome of infection. Int J Obes (Lond) 37: 333-340. [Crossref]
- Zeigler CC, Persson GR, Wondimu B, Marcus C, Sobko T et al. (2012) Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity (Silver Spring) 20: 157-164. [Crossref]
- Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E et al. (2008) Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 3: e3326. [Crossref]
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P et al. (2008) Biofilms in chronic wounds. Wound Repair Regen 16: 37-44. [Crossref]
- Malik A, Mohammad Z, , Ahmad J (2013) The diabetic foot infections: biofilms and antimicrobial resistance. Diabetes Metab Syndr 7: 101-107. [Crossref]
- Watters C, DeLeon K, Trivedi U, Griswold JA, Lyte M et al. (2013) Pseudomonas aeruginosa biofilms perturb wound resolution and antibiotic tolerance in diabetic mice. Med Microbiol Immunol 202: 131-141. [Crossref]
- Kendall RW, Duncan CP, Smith JA, Ngui-Yen JH (1996) Persistence of bacteria on antibiotic loaded acrylic depots. A reason for caution. Clin Orthop Relat Res (329): 273-280. [Crossref]
- Neut D, van de Belt H, Stokroos I, van Horn JR, van der Mei HC et al. (2001) Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J Antimicrob Chemother 47: 885-891. [Crossref]
- Sorli L, Puig L, Torres-Claramunt R, Gonzalez A, Alier A et al. (2012) The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. J Bone Joint Surg Br 94: 249-253. [Crossref]
- Boles BR, , Singh PK (2008) Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A 105: 12503-12508. [Crossref]
- Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A et al. (2014) Diagnosis of periprosthetic joint infection. J Arthroplasty 29: 77-83.
- Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R et al. (2011) Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res 29: 617-622. [Crossref]
- Janz V, Wassilew GI, Hasart O, Tohtz S, Perka C (2013) Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures. J Orthop Res 31: 2021-2024. [Crossref]
- Lass R, Giurea A, Kubista B, Hirschl AM, Graninger W et al. (2014) Bacterial adherence to different components of total hip prosthesis in patients with prosthetic joint infection. Int Orthop 38: 1597-1602. [Crossref]
