p38 MAP Kinase-Mediated Odontogenic Differentiation of Dental Pulp Stem Cells
p38 MAP Kinase-Mediated Odontogenic Differentiation of Dental Pulp Stem Cells
A B S T R A C T
The multipotent nature of dental pulp stem cells (DPSCs) promises regenerative endodontic potentials. Alterations in microenvironment have been shown to control the differentiation phenotypes of DPSCs. Understanding the biological mechanisms and finding the optimal DPSC differentiation protocols are crucial for successful DPSC engineering strategies in pulp and dentin healing. The aim of this study is to identify the role of p38 mitogen-activated protein kinase (p38) under normal and oxygen-deprived conditions (2%) to reveal its effect on odontogenic DPSC differentiation. Human DPSCs were isolated from healthy molars and underwent odontogenic differentiation in regular and osteogenic media treated with SB203580, a p38 inhibitor, for 72 hours, and then swapped with osteogenic media for 21 days under hypoxic condition. Immunochemistry and PCR analysis for the various odontogenic differentiation genes and proteins were performed. Our PCR data demonstrate that p38 inhibition resulted in a significant upregulation in odontogenic gene expressions such as DMP-1, DSPP, RUNX, and OSX in normal conditions. Under hypoxia, this effect was reversed. These results were further supported by DSPP immunohistochemistry. The DSPP expression under hypoxia was significantly weaker compared to the control. Our results indicate that p38 represents a negative regulator of the odontogenic DPSC differentiation in normoxia. Under hypoxia, p38 exerts a positive function of DPSC differentiation. Taken together, we identified the p38 and oxygen level as crucial factors to control odontogenic DPSC differentiation providing their essential roles in designing for successful pulp-dentin complex engineering strategies.
Keywords
DPSC, p38 MAPK, hypoxia, differentiation, odontoblast
Introduction
In recent years in the regeneration field of dentistry, much attention has been given to stem cell biology as they demonstrate the remarkable translational capacity of regenerating dentin and damaged tooth [1, 2]. Dental pulp stem cells (DPSCs) can be acquired relatively with ease compared to other stem cell kinds such as embryonic stem cells (ESCs) and induced pluripotent stem cells. Several studies, including ours, have shown that human DPSCs can successfully differentiate into odontoblast-like cells under special culture conditions [3, 4]. These differentiated cells were able to produce dentin.
Changes in the cultural micro-environment have been shown to affect the phenotypes of stem cells. Extracellular environments, such as extracellular matrix, secreted factors and direct cell to cell contact, have both positive and negative roles in stem cell differentiation and cell fate determination [5, 6]. Oxygen is one of the vital factors and has a variety of functions on stem cell proliferation and differentiation [7-10]. The natural oxygen level in the body is remarkably low – approximately 3% inside the tissue compared to routine 20% oxygen level in the lab tissue culture [11]. Thus, careful consideration of the micro-environmental oxygen level for stem cell engineering strategies needs to be addressed.
Mitogen-activated protein kinases (MAPKs) are an evolutionarily conserved family of enzymes. They are serine/threonine kinases that connect cell-surface receptors to critical regulatory targets within the cell and are essential mediators of various physiological functions in normal as well as disease conditions [12]. p38 MAPKs (p38) are essential mediators of various physiological functions in normal and disease conditions [12]. The p38 has been implicated in roles in tooth development and enamel production as well as in the periodontal tissue remodeling of orthodontic tooth movement [13, 14]. Furthermore, the p38 appears to be involved in dentin regulation. Dentin matrix proteins promoted bone marrow stromal cell differentiation through p38 signaling [15].
The role of p38 in the differentiation of DPSCs, especially under hypoxia, is unknown. Several studies have demonstrated that if the microenvironment is altered, stem cell fate will also be altered [16]. The present study focuses on the molecular signaling of stem cells to differentiate into the appropriate lineage. Our aim is to validate the role of p38-mediated odontogenic DPSC differentiation in the context of oxygen-deprived conditions. Our data will provide an important foundation for future DPSC engineering for dentin regeneration in the damaged tooth.
Methods
I Cell Cultures
DPSCs were collected from immature human healthy molars, and the isolated cells were cultured and expanded using the explant outgrown method [17]. Human third molars were extracted for orthodontics cases in the clinic (UIC protocol #20110129). We also used commercially available cells (Celprogen, #36086). The DPSCs have been evaluated in cultures with the STRO-1, a stem cell marker (IHC and FACS analysis). Our analysis confirms over 99% of cells (398/402, N=6) used for our study were DPSCs. The DPSCs were cultured using explant outgrowth method and will not be used beyond passage 5. DPSCs were cultured in regular media and osteogenic media and treated with p38 antagonist – SB203580 for 72 hours in regular media (regular growth media), and then swapped with osteogenic media for 21 days treated with SB203580. The medium was changed every 3 days. The procedure was done under hypoxia (2% oxygen) and normoxia (20% oxygen) conditions. The DPSCs were cultured at 37°C and 5% CO2 in regular media and osteogenic media. DPSCs between 2nd and 4th passages were used throughout the study. All experimental protocols used for this study were in accordance with the guidelines according to the Institutional Animal Care and Use Policy and approved by the IRB Protocol Committee at the University of Illinois at Chicago.
II Quantitative PCR Analysis
Isolated DPSCs were cultured according to the differentiation protocol above in 6 wells plate with triplicates at 40,000 - 50,000 cells/well concentration for the treatment of the C5aR inhibitor W-54011. Total mRNA was extracted with 0.8 ml Trizol (Invitrogen, CA) and analysed using the Fisher Scientific NanoDrop 2000 device. The cDNA samples were analysed using the Applied Biosystems SYBR green reagent system according to the manufacture protocol. The list of primers and their sequences used are provided in (Table 1).
Table 1: List of primers and their sequences used.
|
CDC42 forward |
5’-GGC GGA GAA GCT GAG GAC AAG-3’ |
|
CDC42 Reverse |
5’-AGC GGT CGT AGT CTG TCA TAA TCC TC-3’ |
|
DMP1 forward |
5’-CAC TCA AGA TTC AGG TGG CAG-3’ |
|
DMP1 reverse |
5’-TCT GAG ATG CGA GAC TTC CTA AA-3’ |
|
P38 forward |
5’-ACT CAG ATG CCG AAG ATG AAC-3’ |
|
P38 reverse |
5’-GTG CTC AGG ACT CCA TCT CT-3’ |
|
MAPK forward |
5’-GGW GAY GGT ACW TAY GGW TC- 3’ |
|
MAPK reverse |
5’-AAG YTT RTA WCC YTC WGG CCA-3’ |
|
BMP1 forward |
5′-CAG TCC TTT GAG ATT GAG CGC-3′ |
|
BMP1 reverse |
5′-TGC TGC TCT CAC TGT GC CC-3′ |
|
FGF forward |
5′-AAG GGC TTT TAT ACG GCT CG-3′ |
|
FGF reverse |
5′-CCC ACA AAC CAG TTC TTC TCC-3′ |
|
DSPP forward |
5’-CTG TTG GGA AGA GCC AAG ATA AG -3′ |
|
DSPP reverse |
5’-CCA AGA TCA TTC CAT GTT GTC CT -3′ |
|
RUNX2 forward |
5′-CCT GAA CTC TGC ACCAAG TG-3′ |
|
RUNX2 reverse |
5′-GAG GTG GCA GTG TCA TCA TC-3′ |
|
OSX forward |
5’-GGC ACA AAG AAG CCG TAC TC-3′ |
|
OSX reverse |
5’-GCC TTG TAC CAG GAG CCA TA-3′ |
|
JNK forward |
5’-CTC CAG CAC CCA TAC ATC AAC-3’ |
|
JNK reverse |
5’-TCA GTT CTT TCC ACT CCT CTA TTG-3’ |
|
GAPDH forward |
5’-GGC ATC CAC TGT GGT CAT GAG -3′ |
|
GAPDH reverse |
5’-TGC ACC ACC AAC TGC TTA GC -3′ |
III Alizarin Red Staining
Fourth passage DPSCs subject to their respective control or treatment were fixed with 10% paraformaldehyde for 15 minutes then washed three times with distilled water. 1 mL of Alizarin red dye was added for 20 minutes then washed four times with deionized water with gentle rocking to remove any additional Alizarin red. 1 mL of 10% acetic acid was added to prevent the cells from drying. The cells were then subject to visual inspection and imaging.
IV Immunohistochemistry
Differentiating and differentiated DPSCs were fixed and saturated. Then, cells were incubated for 4 hours with rabbit anti-p38 (1:250, Sigma) and mouse anti-DSPP (1:500, Sana Cruz) or their respective control isotypes. Finally, cells were treated for 40 minutes with a mix of Alexa Fluor-594 anti-mouse IgG, Alexa Fluor-488 anti-rabbit IgG (2 μg/mL) and/or DAPI (1 μg/mL). The coverslips were mounted, and images were taken using a Leica DMI6000 B microscope. Fluorescence staining was statistically determined by using the integrated density of each condition using ImageJ 1.49v software.
V Statistical Analysis
For quantification of histology and immunohistochemistry staining intensity, ImageJ was used. Fixed areas of 1mm X 1mm or 2mm X 2mm were selected to analyze the number of differentiated cells. The statistical significance was demonstrated using the Student’s t-test (P < 0.05 was considered significant).
Results
I Odontogenic Lineage Cell Markers Such as DSPP, DMP-1 and RUNX2 are Expressed in the Differentiating and Differentiated Cells from DPSCs
To demonstrate specific in vitro role of p38 in DPSC odontogenic differentiation both in normal and oxygen-deprived conditions, isolated DPSCs were differentiated into odontoblast-like cells using p38 inhibitor SB203580 by our modified differentiation protocol. The human DPSCs were acquired from molars and cultured using regular growth and osteogenic medium for 24 days. A general p38 inhibitor SB203580 was treated from the differentiation day (D) 1 to D24 (for more details, see a timeline in Figure 1A). The homogeneous purity of the DPSC population was checked and validated in our previous reports [3, 4].
We next analysed the cell identity after full differentiation using several odontoblast lineage markers by real-time PCR and immunohistochemistry. The differentiated cells demonstrated odontoblast-like characteristics as they expressed odontogenic cell markers such as dentin matrix protein (DMP)-1, runt-related transcription factor 2 (RUNX2) and dentin sialophosphoprotein (DSPP) mRNA (Figure 2) and DSPP protein (Figure 1C). These are well-known odontoblast lineage-specific genes [18, 19]. Alizarin mineralization staining also confirmed a calcification (Figure 1B). Thus, our comprehensive odontogenic marker analysis demonstrated a successful odontogenic differentiation of DPSCs both in normal and oxygen-deprived conditions.
Figure 1: A schematic timeline representation of odontogenic DPSC differentiation and p38 is expressed in DPSC-derived differentiating cells.
A) DPSCs were acquired from healthy molars using the explant outgrowth method. After reaching confluency, DPSCs were cultured and treated with regular and osteogenic media for 4 days. C5aR inhibitor SB203580 was added to the osteogenic media from the differentiation day 1 to day 24. The medium was replaced every 3 days. Alizarin red staining demonstrates a successful calcium mineralization, which is the odontoblast characteristics, both in the SB203580 treatment and control groups. Representative image of differentiating DPSCs at day (D) 4. B) Alizarin red staining shows a successful calcium compound detection both in the SB203580 treatment and control groups. C-E) Double immunofluorescence staining with anti-DSPP and p38 (green) in DPSC-derived cells at day 20 demonstrates that DSPP-immunoreactive cells (red) do express p38 (green) confirming a successful odontogenic differentiation and p38 expression in the DPSCs-derived cells. Scale bar: 50 μm.
II Differentiating DPSCs express p38
We next identified the p38 expression in DPSCs-derived differentiating cells. Double fluorescence immunostaining with anti-DSPP and p38 antibodies demonstrated that the differentiating cells express both DSPP and p38 confirming both a successful odontogenic differentiation and p38, expression (Figures 1C-1E).
III p38 Inhibition Resulted in a Significant Upregulation in Odontogenic Differentiation Gene Expression
PCR gene expression analysis of p38, fibroblast growth factor (FGF), bone morphogenetic protein 1 (BMP1), DMP-1, RUNX2, Cell Division Cycle 42 (CDC42), c-Jun N-terminal kinases (JNK), ON in the SB203580 treatment group compared to control (as represented value ‘1’ of respective genes). The data demonstrate significant upregulation of odontoblast lineage genes such as DSPP, RUNX2 and DMP-1 in the W-54011 treatment group compared to control (Figure 2).
Figure 2: PCR analysis of DPSC-derived odontoblast-like cells in normoxia.
Real-time PCR analysis of p38, MAPK, FGF, BMP1, DMP-1, RUNX2, CDC42, JNK, ON in the SB203508 treatment group compared to control (calculated ‘1’ value of respective genes expression). The analysis reveals a significant upregulation of odontoblast lineage genes such as DSPP, RUNX2 and DMP-1 in the SB203508 treatment group.
IV p38 Inhibition Under Hypoxia Resulted in a Significant Reduction in Odontogenic Differentiation Gene Expression
Under hypoxia with the p38 inhibition, odontogenic lineage genes such as DMP-1 and DSPP expression is significantly decreased compared to control (without p38 inhibition) and normoxia groups (with p38 inhibition) (Figure 3).
V DSPP Expression in the Differentiated Odontoblast-Like Cells Is Decreased with p38 Inhibition and This Effect Is Reversed in Hypoxia
To further support gene expression data, we next performed the DSPP protein immunostaining with DPSCs-differentiated cells. In normal cells, without hypoxia induction and SB203580 treatment, the DDPP expression is evenly present in the cytoplasm of the differentiated cells (Figures 4A-4F). However, the DSPP expression is significantly upregulated in the SB203580 treatment group (Figures 4B & 4D), suggesting p38`s negative role in odontogenic DPSC differentiation. Consistent with the PCR data (Figure 3), this effect was completely reversed in the oxygen-deprived condition. DSPP expression is significantly downregulated in the differentiated cells under hypoxia (Figures 4C & 4G).
The DSPP expression staining intensity was quantified using ImageJ software. The SB203580 treatment under hypoxia resulted in a significant downregulation in DSPP expression of the differentiated cells compared to the control normoxia group (blue: 1.56 ± 0.08, N=4 versus orange: 0.73 ± 0.06, N=5, p<0.005). Taken together, our data demonstrate that p38 has a negative role in odontogenic DPSC differentiation, and this effect was reversed in oxygen-deprived condition.
Figure 3: PCR analysis of DPSC-derived odontoblast-like cells in hypoxia.
Real-time PCR analysis of p38, MAPK, AP1, CDC42, BMP1, FGF, ON, DSPP, RUNX2, OSX, DMP-1 in the SB203508 treatment group compared to control (calculated ‘1’ value of respective genes expression) in oxygen-deprived condition. The analysis reveals a significant decrease of odontoblast lineage genes such as DSPP and DMP-1 in the SB203508 treatment group compared to control demonstrating the hypoxia induction reverse the odontogenic differentiation phenotype of p38 inhibition.
VI Hypoxia Induction Has Little Effect on Odontoblast-Like Cell Proliferation
To demonstrate whether the change of odontogenic gene expressions is due to the increased or decreased cell number during DPSC differentiation, we next examined the proliferation capacity of the differentiated cells. The number of DSPP-positive cells was counted and analysed in a fixed area of 1 X 1 mm2 in the captured images. The number of the differentiated cells did not show a significant difference between the normoxia (1.23 ± 0.14, N=4) and hypoxia (1.04 ± 0.17, N=4, p<0.05) groups (Figure 4G). Our analysis demonstrates that p38 signaling and hypoxia induction have little effect on the odontogenic proliferation of DPSCs.
Figure 4: DSPP expression in the DPSC derived odontoblast-like cells in normoxia and hypoxia.
A-F) Immunofluorescence double immunostaining with anti-DSPP (red) at 26 days of differentiation demonstrating the control (A, D), SB203580 treatment group in normoxia (B, E) and SB203580 treatment group under hypoxia (C, F). DMP-1 is expressed uniformly in the cytoplasm of the DPSCs-derived cells both in normal (A, D) and hypoxia group (B, E). p38 inhibition induced an upregulation of DSPP expression (B, E). However, the DSPP expression is significantly downregulated in the SB203580 treatment group in hypoxia (C, F).
G) DSPP fluorescence intensity was quantified using image J software (Left) and the number of the cells between the normoxia and hypoxia were counted and calculated as the control value “1”. The SB203580 treatment group in hypoxia resulted in a downregulation of DSPP expression of the differentiated cells (blue: 1.56 ± 0.08, N=4 versus orange: 0.73 ± 0.06, N=5, *** p<0.005). The number of the cells did not show a meaningful difference between the normoxia (1.23 ± 0.14, N=4) and hypoxia (1.04 ± 0.17, N=4, p<0.05) groups.
Discussion
In this study, we first successfully differentiated our isolated DPSCs into odontoblast-like cells. Our results demonstrate that p38 constitutes a negative regulator of odontogenic DPSC differentiation as the p38 inhibition induces a significant upregulation of odontogenic lineage gene expression such as DMP1, RUNX2 and DSPP. The hypoxia induction showed opposite results. Our results confirm that the physical changes in microenvironment, such as hypoxia, are important factors in altering the differentiation properties of DPSCs.
Traditionally, immature necrotic teeth with apical periodontitis have been treated with apexification procedures using either long-term calcium hydroxide treatment or the MTA barrier method. A major disadvantage of apexification is that there is no potential for further development of the immature root end in terms of dentin thickness as well as root length. Additionally, long-term treatment with calcium hydroxide may predispose the already fragile immature root to fracture. In 2004, Banchs and Trope proposed a clinical protocol that is similar to the current AAE guidelines for revascularization based on observations of revascularization of reimplanted teeth, disinfection of the necrotic canal space, and induction of a blood clot into the canal [20]. Their study also found successful results. Today, regenerative endodontic therapy is recommended as an alternative treatment to the traditional apexification procedures, and regenerative potential of DPSC promises a translational capacity of repairing damaged necrotic teeth and pulp.
The roles of p38 in several stem cell differentiation and proliferation have been studied. However, the results of p38 in osteogenic and chondrogenic differentiation from mesenchymal stem cells (MSCs) have been controversial. Both positive and negative roles of p38 in bone tissue differentiation have been reported [21-23]. It appears that the role of p38 in stem cell differentiation is complicated, and many factors, including the cell culture condition, timing and dose of inhibitor treatment, seem to play vital roles in MSCs differentiation.
The triad of tissue engineering includes stem cells, a physical scaffold and signaling molecules. But even with an appropriate source of stem cells, the differentiation lineage may not be appropriate due to an altered microenvironment. Once a canal becomes infected, the microenvironment is changed; and once the canal is disinfected with antimicrobials, the microenvironment is also changed. One example of this is that in the presence of LPS, stem cells from the apical papilla introduced into root canal segments were found to shift towards osteogenic phenotype as opposed to the odontogenic phenotype [24]. Many other authors have found that if the microenvironment is altered, stem cell fate will also be altered [5-11]. Thus, careful control of the microenvironment is vital to the success of regeneration strategies. The present study focuses on the molecular signaling of stem cells for their differentiation into the appropriate lineage.
There have been a number of studies revealing the oxygen`s role in maintaining undifferentiated stem cell status and stem cell differentiation/proliferation. The ESCs can maintain a longer period of undifferentiated status under low oxygen [9]. Human ESCs and MSC-derived cells showed more bone and cartilage differentiation in oxygen-deprived conditions [7, 8]. There were also more bone cells and elevated markers of osteogenesis [7]. Human ESCs in low oxygen tension had increased ability to produce type I and II collagen and had different CD markers present on their cell surfaces [8]. Finally, hypoxia has been used to promote differentiation into various cell lines while also having an influence on the production of growth factors involved in angiogenesis [11].
Surprisingly, our study demonstrates that the hypoxia induction oppositely regulates differentiation gene expression compared to normal conditions. This observation is consistent with our pilot study that immune complement fragment C5a plays a positive role in odontogenic differentiation of DPSCs; however, this effect was reversed in oxygen-deprived condition. C5a receptor blocker induced a significant downregulation of odontogenic differentiation genes such as DMP1, ON, RUNX2, DSPP in normal condition, but under hypoxia, these gene expressions were significantly increased (Pasiewicz et al., unpublished observation). We do not understand the exact mechanism of this phenotype change. Further in detailed molecular pathway analysis is needed. Previous studies have demonstrated that hypoxia induction increases the proliferation of several types of stem cells [7-9, 25]. However, either p38 inhibition or hypoxia induction did not result in a meaningful change in DPSCs proliferation. It seems that several environmental factors, including the differentiation stage, stem cell kinds, degrees of hypoxia and culture conditions, are crucial factors in the differentiation phenotype determination.
In this study, we report that the p38 and oxygen levels are important regulators in the differentiation of DPSCs both in normal and hypoxia conditions. Controlling the microenvironment in stem cell differentiation is a vital strategy for successful dentin repair. Our data will provide an important basis for future DPSC-mediated pulp-dentin regeneration engineering.
Acknowledgement
This study was supported by the NIH/NIDCR grant (to S. Alapati grant no. DE019514).
Author Contributions
Cheng W, Valverde Y, Chung SH contributed to conception, design, data acquisition (immunohistochemistry), analysis, and interpretation, drafted and critically revised the manuscript. Lee NS, Marzban H contributed data acquisition and critically revised the manuscript. Alapati S provided DPSCs and human tooth sections for the study and also contributed to design, data analysis (DPSC differentiation), and interpretation the results. All authors reviewed and gave final approval.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 28, May 2020Accepted: Wed 10, Jun 2020
Published: Fri 03, Jul 2020
Copyright
© 2023 Seung Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2020.02.03
Author Info
William Cheng Yessenia Valverde Nam-Seob Lee Hassan Marzban Seung Chung SB Alapati
Corresponding Author
Seung ChungDepartment of Oral Biology, University of Illinois at Chicago, Chicago, Illinois , USA
Figures & Tables
Table 1: List of primers and their sequences used.
|
CDC42 forward |
5’-GGC GGA GAA GCT GAG GAC AAG-3’ |
|
CDC42 Reverse |
5’-AGC GGT CGT AGT CTG TCA TAA TCC TC-3’ |
|
DMP1 forward |
5’-CAC TCA AGA TTC AGG TGG CAG-3’ |
|
DMP1 reverse |
5’-TCT GAG ATG CGA GAC TTC CTA AA-3’ |
|
P38 forward |
5’-ACT CAG ATG CCG AAG ATG AAC-3’ |
|
P38 reverse |
5’-GTG CTC AGG ACT CCA TCT CT-3’ |
|
MAPK forward |
5’-GGW GAY GGT ACW TAY GGW TC- 3’ |
|
MAPK reverse |
5’-AAG YTT RTA WCC YTC WGG CCA-3’ |
|
BMP1 forward |
5′-CAG TCC TTT GAG ATT GAG CGC-3′ |
|
BMP1 reverse |
5′-TGC TGC TCT CAC TGT GC CC-3′ |
|
FGF forward |
5′-AAG GGC TTT TAT ACG GCT CG-3′ |
|
FGF reverse |
5′-CCC ACA AAC CAG TTC TTC TCC-3′ |
|
DSPP forward |
5’-CTG TTG GGA AGA GCC AAG ATA AG -3′ |
|
DSPP reverse |
5’-CCA AGA TCA TTC CAT GTT GTC CT -3′ |
|
RUNX2 forward |
5′-CCT GAA CTC TGC ACCAAG TG-3′ |
|
RUNX2 reverse |
5′-GAG GTG GCA GTG TCA TCA TC-3′ |
|
OSX forward |
5’-GGC ACA AAG AAG CCG TAC TC-3′ |
|
OSX reverse |
5’-GCC TTG TAC CAG GAG CCA TA-3′ |
|
JNK forward |
5’-CTC CAG CAC CCA TAC ATC AAC-3’ |
|
JNK reverse |
5’-TCA GTT CTT TCC ACT CCT CTA TTG-3’ |
|
GAPDH forward |
5’-GGC ATC CAC TGT GGT CAT GAG -3′ |
|
GAPDH reverse |
5’-TGC ACC ACC AAC TGC TTA GC -3′ |

A) DPSCs were acquired from healthy molars using the explant outgrowth method. After reaching confluency, DPSCs were cultured and treated with regular and osteogenic media for 4 days. C5aR inhibitor SB203580 was added to the osteogenic media from the differentiation day 1 to day 24. The medium was replaced every 3 days. Alizarin red staining demonstrates a successful calcium mineralization, which is the odontoblast characteristics, both in the SB203580 treatment and control groups. Representative image of differentiating DPSCs at day (D) 4. B) Alizarin red staining shows a successful calcium compound detection both in the SB203580 treatment and control groups. C-E) Double immunofluorescence staining with anti-DSPP and p38 (green) in DPSC-derived cells at day 20 demonstrates that DSPP-immunoreactive cells (red) do express p38 (green) confirming a successful odontogenic differentiation and p38 expression in the DPSCs-derived cells. Scale bar: 50 μm.
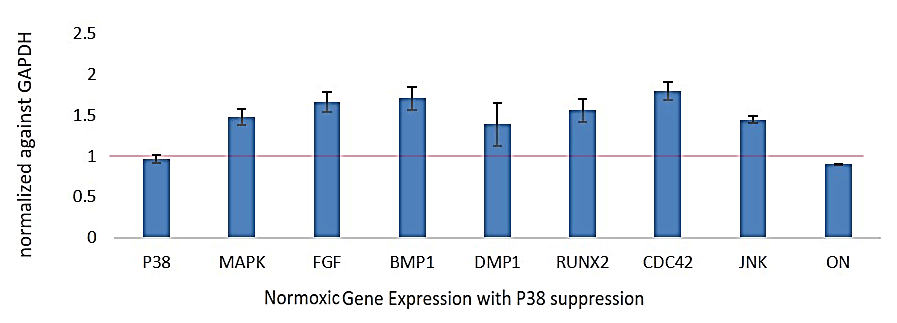
Real-time PCR analysis of p38, MAPK, FGF, BMP1, DMP-1, RUNX2, CDC42, JNK, ON in the SB203508 treatment group compared to control (calculated ‘1’ value of respective genes expression). The analysis reveals a significant upregulation of odontoblast lineage genes such as DSPP, RUNX2 and DMP-1 in the SB203508 treatment group.

Real-time PCR analysis of p38, MAPK, AP1, CDC42, BMP1, FGF, ON, DSPP, RUNX2, OSX, DMP-1 in the SB203508 treatment group compared to control (calculated ‘1’ value of respective genes expression) in oxygen-deprived condition. The analysis reveals a significant decrease of odontoblast lineage genes such as DSPP and DMP-1 in the SB203508 treatment group compared to control demonstrating the hypoxia induction reverse the odontogenic differentiation phenotype of p38 inhibition.

A-F) Immunofluorescence double immunostaining with anti-DSPP (red) at 26 days of differentiation demonstrating the control (A, D), SB203580 treatment group in normoxia (B, E) and SB203580 treatment group under hypoxia (C, F). DMP-1 is expressed uniformly in the cytoplasm of the DPSCs-derived cells both in normal (A, D) and hypoxia group (B, E). p38 inhibition induced an upregulation of DSPP expression (B, E). However, the DSPP expression is significantly downregulated in the SB203580 treatment group in hypoxia (C, F). G) DSPP fluorescence intensity was quantified using image J software (Left) and the number of the cells between the normoxia and hypoxia were counted and calculated as the control value “1”. The SB203580 treatment group in hypoxia resulted in a downregulation of DSPP expression of the differentiated cells (blue: 1.56 ± 0.08, N=4 versus orange: 0.73 ± 0.06, N=5, *** p<0.005). The number of the cells did not show a meaningful difference between the normoxia (1.23 ± 0.14, N=4) and hypoxia (1.04 ± 0.17, N=4, p<0.05) groups.
References
- Mabel M Cordeiro, Zhihong Dong, Tomoatsu Kaneko, Zhaocheng Zhang, Marta Miyazawa et al. (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34: 962-969. [Crossref]
- Koichiro Iohara, Kiyomi Imabayashi, Ryo Ishizaka, Atsushi Watanabe, Junichi Nabekura et al. (2011) Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A 17: 1911-1920. [Crossref]
- Michael Boyle, Crystal Chun, Chelsee Strojny, Raghuvaran Narayanan, Amelia Bartholomew et al. (2014) Chronic inflammation and angiogenic signaling axis impairs differentiation of dental-pulp stem cells. PLoS One 9: e113419. [Crossref]
- Taneka D Jones, Hamed Naimipour, Shan Sun, Michael Cho, Satish B Alapati (2015) Mechanical changes in human dental pulp stem cells during early odontogenic differentiation. J Endod 41: 50-55. [Crossref]
- Tali Ra'em, Smadar Cohen (2012) Microenvironment design for stem cell fate determination. Adv Biochem Eng Biotechnol 126: 227-262. [Crossref]
- Francesca Gattazzo, Anna Urciuolo , Paolo Bonaldo (2014) Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840: 2506-2519. [Crossref]
- D P Lennon, J M Edmison, A I Caplan (2011) Cultivation of rat marrow derived mesenchymal stem cells in reduced oxygen tension: effect on in vitro and in vivo osteochondrogenesis. J Cell Phys 187: 345-355. [Crossref]
- E J Koay, K A Athanasiou (2008) Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis and Cartilege 16: 1450-1456. [Crossref]
- S M Prasad, M Czepiel, C Cetinkaya, K Smigielska, S C Weli et al. (2009) Continous hypoxic culturing maintains activation of Notch and allows long-term propogation of human embryonic stem cells without spontaneous differentiation. Cell Prolif 42: 63-74. [Crossref]
- Hamid Abdollahi, Lisa J Harris, Ping Zhang, Stephen McIlhenny, Vikram Srinivas et al. (2011) The role of hypoxia in stem cell differentiation and therapeutics. J Surg Res 165: 112-117. [Crossref]
- Marie Csete (2005) Oxygen in the cultivation of stem cells. Ann NY Acad Sci 1049: 1-8. [Crossref]
- Ana Cuenda, Simon Rousseau (2007) p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773: 1358-1375. [Crossref]
- Matthew B Greenblatt, Jung-Min Kim, Hwanhee Oh, Kwang Hwan Park, Min-Kyung Choo et al. (2015) p38α MAPK is required for tooth morphogenesis and enamel secretion. J Biol Chem 290: 284-295. [Crossref]
- Liping Jiang, Zhen Tang (2018) Expression and regulation of the ERK1/2 and p38 MAPK signaling pathways in periodontal tissue remodeling of orthodontic tooth movement. Mol Med Rep 17: 1499-1506. [Crossref]
- Yan Yu, Lijuan Wang, Jinhua Yu, Gang Lei, Ming Yan et al. (2014) Dentin matrix proteins (DMPs) enhance differentiation of BMMSCs via ERK and P38 MAPK pathways. Cell Tissue Res 356: 171-182. [Crossref]
- Amy J Wagers (2012) The stem cell niche in regenerative medicine. Cell Stem Cell 10: 362-369. [Crossref]
- Yessenia Valverde, Raghuvaran Narayanan, Satish B Alapati, Fanny Chmilewsky, Chun-Chieh Huang et al. (2018) PARP-1 inhibition enhances brain-derived neurotrophic factor secretion in dental pulp stem cells-derived odontoblast-like cells. J Endod 44: 1121-1125. [Crossref]
- K Gu, S Chang, H H Ritchie, B H Clarkson, R B Rutherford (2000) Molecular cloning of a human dentin sialophosphoprotein gene. Eur J Oral Sci 108: 35-42. [Crossref]
- A Almushayt, K Narayanan, A E Zaki, A George (2006) Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther 7: 611-620. [Crossref]
- Francisco Banchs, Martin Trope (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30: 196-200. [Crossref]
- Brent E Bobick, Alexander I Matsche, Faye H Chen, Rocky S Tuan (2010) The ERK5 and ERK1/2 signaling pathways play opposing regulatory roles during chondrogenesis of adult human bone marrow-derived multipotent progenitor cells. J Cell Physiol 224: 178-186. [Crossref]
- J Li, Z Zhao, J Liu, N Huang, D Long et al. (2010) MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif 43: 333-343. [Crossref]
- Hye-Joung Kim, Gun-Il Im (2010) The effects of ERK1/2 inhibitor on the chondrogenesis of bone marrow- and adipose tissue-derived multipotent mesenchymal stromal cells. Tissue Eng Part A 16: 851-860. [Crossref]
- Lakshmi Vishwanat, Rose Duong, Koyo Takimoto, Linda Phillips, Claudia O Espitia et al. (2017) Effect of Bacterial Biofilm on the Osteogenic Differentiation of Stem Cells of Apical Papilla. J Endod 43: 916-922. [Crossref]
- Preeti Malladi, Yue Xu, Michael Chiou, Amato J Giaccia, Michael T Longaker (2006) Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol 290: 1139-1146. [Crossref]
