Journals
Overexpression of BMP-2 in mesenchymal stem cells with amplifying virus-like particles
A B S T R A C T
There are numerous strategies under development to supplement or replace bone grafts. Among these synthetic options, growth factors such as bone morphogenetic protein (BMP-2) are often used. However, current burst delivery strategies have been associated with a number of complications. Transplanting cells engineered to over-express osteoinductive proteins over a longer period of time is a possible solution to this problem. In this study, we demonstrated the use of a parainfluenza virus 5 (PIV-5) vector (amplifying virus-like particles, AVLP) to induce expression of exogenous proteins in mesenchymal stem cells (MSC). We obtained extended expression of enhanced green fluorescent protein eGFP in transduced MSCs, as well as selection and maintenance of eGFP+ cells. We also attained expression of BMP-2 on a shorter timescale using this method. We conclude that AVLP is a promising tool for genetic engineering, both in regenerative medicine and other applications.
K E Y W O R D S
mesenchymal stem cells, parainfluenza virus 5, genetic therapy, bone morphogenetic protein-2
I N T R O D U C T I O N
Bone tissue is well known for its remarkable healing abilities, but there are instances in which these mechanisms are not sufficient to bridge large defects or gaps naturally. Historically, bone grafts have been used to aid in bone regeneration when needed. Allografts and autografts are the most popular bone grafts used, accounting for over 2 million procedures per year [1]. Allografts, derived from other individuals of the same species, are potential sources of disease transmission and can fail to integrate with the host’s existing bone [2, 3]. Autografts, which are derived from the patient themselves, are the current gold standard, but are the most difficult to obtain and have the risk of donor site morbidity [2, 4]. To overcome the challenges associated with allografts and autografts there have been numerous attempts to develop synthetic bone graft substitutes. One of the most widespread strategies in developing these substitutes is the incorporation of growth factors and cells that encourage bone formation [5, 6].
Bone morphogenetic protein 2 (BMP-2) is an osteoinductive growth factor commonly used in bone substitute applications. The recombinant human protein (rhBMP-2) is FDA-approved and used clinically in combination with a collagen sponge for spinal fusions and tibial shaft fractures [7]. BMP-2 has reduced the rate of secondary intervention and enhanced fracture healing in many orthopedic applications, including facial reconstruction, maxillary sinus floor augmentation, long bone non-unions, tibial fractures, and lumbar fusions [8, 9]. Despite its efficacy, rhBMP-2 has a very short half-life requiring a high dose for efficacy [10, 11]. Clinically relevant doses of BMP-2 in rats induced the formation of structurally abnormal bone as well as inflammation [12]. In humans, this amount of BMP-2 has been associated with ectopic bone formation and increased cancer rates [13, 14]. Additionally, rhBMP-2 is often an expensive therapeutic option, costing upwards of $3500 per kit, of which several may be used per patient [15].
To reduce comorbidities and cost associated with BMP-2 treatment, numerous groups have investigated the transplantation of BMP-expressing cells as an alternative to this treatment [16]. Our group has demonstrated comparable bone growth in a rat femoral defect model between the collagen sponge delivery method and mesenchymal stem cells (MSCs) transduced to overexpress BMP-2 using a lentiviral vector (under review). Despite BMP expression in lentiviral and adenoviral vector-transduced MSCs, technical drawbacks associated with these gene therapy delivery systems reduce the likelihood that this treatment option would advance to clinical use for bone regeneration [17]. For example, commonly used techniques for lentiviral transduction inhibit proliferation of the transduced cells thus negating the possibility of subculture, while the vector’s expense precludes transducing each new lot [18, 19]. Additionally, lentiviral vectors have been subject to a number of safety concerns regarding the possibility of off-target effects and oncogene activation [20]. While adenoviral vectors do not have the safety, issues associated with lentiviral ones, when applied to BMP-2 expression their protein yield is comparatively low [21, 22]. Therefore, a safer and lower-cost vector that allows for cell proliferation while inducing BMP-2 expression comparable to the lentiviral vector would make for a more viable therapy.
Parainfluenza virus 5 (PIV-5) is a paramyxovirus with negative-sense single stranded RNA [23]. It is a 150-300 nm diameter spherical, enveloped virus 150-300 nm in diameter that is rarely pathogenic in immune-competent individuals despite infecting a wide variety of cells and species [24, 25]. Rather than integrating into the host genome, PIV-5 creates an RNA episome within the host cell’s cytoplasm. Amplifying virus-like particles (AVLP) have been developed from PIV-5 by our group to express viral proteins in transduced cells [26-29]. This prior success indicates that AVLP may be able to find wider use as a viral vector.
Musculoskeletal gene therapy will benefit from more efficient vectors that generate fewer off target effects. We addressed this need by initially testing AVLP technology and determining whether an AVLP-BMP vector would induce comparable or greater BMP-2 expression in MSCs compared to other vectors, while allowing for selection and maintenance of BMP-2 overexpressing cells. We found that AVLP can be used to reliably express eGFP at high levels over time in MSCs and can also be used to express BMP-2 over shorter periods of time.
Methods
AVLP generation
Generation of amplifying virus-like particles (AVLP) from PIV5 was performed as described by Wei et al. [29]. Briefly, a plasmid with the full-length genome of PIV-5 was used to construct the PIV5 AVLP vector (pAVLP) (Fig 1A). The AVLP vector contains the nucleoprotein, phosphoprotein, V protein, and L RNA-dependent RNA polymerase of PIV-5 (Fig. 1A). Genes for either eGFP or BMP-2 were inserted into pAVLP between the V/P and hygromycin genes, creating AVLP–eGFP or AVLP–BMP2.
Figure 1: AVLP construct and transduction of MSCs. (A)The AVLP construct contains the nucleoprotein (NP), phosphoprotein (P), V protein (V), and L RNA-dependent RNA polymerase (L) from PIV-5, as well as the hygromycin-B resistance gene and either BMP-2 or eGFP. (B) Human umbilical MSCs were successfully transduced with AVLP-eGFP at a high efficiency as shown 48 hours post-transduction. (C) Human umbilical mesenchymal stem cells (huMSC) were transduced with AVLP-BMP and incubated for up to 120 hours. Both cell types secreted highly variable amounts of BMP-2. n=3, mean +/- SEM.
Cell Culture and Transduction
Human umbilical MSCs (huMSC, Lifeline Cell Technology, Frederick MD, Sciencell, Carlsbad CA) were plated at 5000 cells/cm2 on tissue culture flasks in complete medium and allowed to grow to 80-90% confluency (20,000–25,000 cells/cm2) in 5% CO2 at 37C. One day prior to infection with AVLP, the cells were removed from the plate using 0.05% Trypsin-EDTA (Gibco) then counted with a TC20 automated cell counter (Bio-Rad Laboratories) using Trypan Blue according to the manufacturer’s instructions. Viability of the cell population was determined as the fraction of cells unstained by Trypan Blue to the total number of cells. They were then plated at 26,000 cells/cm2 in complete growth medium and incubated overnight at 37C and 5% CO2. Next, the cells were infected at 5 MOI with either AVLP-eGFP or AVLP-BMP2 in 0.25 mL of complete medium per cm2 of growth area and incubated for 24 hours in 5% CO2 at 37C. Finally, the infection medium was changed and replaced with fresh complete medium, after which the cells were used according to the particular experiment.
BMP-2 Expression
Conditioned culture medium was collected from separate wells at 48, 72, 96, and 120 hours post-transduction and frozen at -80C. The collected medium was later analyzed for BMP-2 by ELISA (R&D Systems).
AVLP-hygro-eGFP selection
At 24 hours post-transduction, two wells per group of huMSC (AVLP-eGFP) received either complete medium or selection medium (complete medium with 50 µg/mL hygromycin B), as did two wells per group of huMSC (n=3). Additionally, two wells per group of either huMSC (AVLP-eGFP) or huMSC were harvested and analyzed for GFP expression by flow cytometry.
Subsequently, cells were maintained in culture for 1 week with medium changed every 2 days using either growth or selection medium. At 8 days post-transduction, all cells were harvested, with a portion of each group being subcultured at 5000 cells/cm2 and the rest being analyzed for eGFP expression via flow cytometry. The subcultured cells were propagated in either selection or growth medium. Upon reaching 80% confluence, they were again harvested, and some were subcultured and the rest analyzed using flow cytometry. This was repeated until all cells had been subcultured 5 times after transduction.
Flow cytometry
All flow analysis was performed using a CyAn ADP (Beckman Coulter, Hialeah, Florida) flow cytometer. Cell suspensions were gated by forward and side scatter to exclude small debris. Gates for eGFP were set such that huMSC cultured in parallel with huMSC (AVLP-eGFP) were 1% positive (Figure 2). FlowJo software (Treestar, Inc., Ashland, Oregon) was used in flow data analysis.
AVLP-BMP2 selection
At 24 hours post-transduction, two wells per group of huMSC (AVLP-BMP2) received either complete medium or selection medium (complete medium with 50 µg/mL hygromycin B), as did two wells per group of huMSC (n=3). Also, at 24 hours post-transduction, the medium of two wells per group of either huMSC (AVLP-BMP2) or huMSC was collected and stored at -80C.
Subsequently, cells were cultured for 1 week with media changes every 2 days using the appropriate medium. After this, all cells were harvested, with a portion of each group being subculture at 5000 cells/cm2 and another portion subculture at 26000 cells/cm2. The cells plated at 26000 cells/cm2 were incubated for 24 hours, after which their medium was collected and stored at -80C. The cells plated at 5000 cells/cm2 grew in either selection or growth medium until reaching 80% confluence when they were again harvested, counted, and split into continued culture and media collection. This was repeated until all cells had been subculture 5 times after transduction. All collected media was assayed for BMP-2 expression via BMP-2 ELISA.
Figure 2: Flow cytometry of huMSC (AVLP-eGFP). Flow cytometry plot of human umbilical mesenchymal stem cells (huMSCs) 48 hours post-transduction with AVLP-eGFP (A), 7 days after selection with hygromycin B (B), and after sequential expansion for 5 passages (C).
Statistics and Calculations
Population doubling level (PDL) was calculated as described by Kruse et al and its equation shown in Equation 1 [30]. The difference in population doubling levels between passages is referred to as population doublings and was used to describe cell growth.
PDL=X+3.322(logY-logI)
Equation 1: Population Doubling Level. Where PDL is the population doubling level, X is the initial population doubling level, I is the initial cell number plated in the culture vessel, and Y is the final cell number at the end of the growth period. In this study, the end of the growth period corresponded with 80% confluence unless otherwise noted.
Unless otherwise noted, all data were analyzed via two-way ANOVA with Bonferroni’s test as appropriate using GraphPad Prism software. Significance was determined as p < 0.05.
Results
At 48 hours post-transduction with 5 MOI AVLP-eGFP, huMSC (AVLP-eGFP) were positive for eGFP (Fig. 1B). BMP-2 ELISA of media collected from AVLP-BMP2 transduced MSCs at 48, 72, 96, and 120 hours post-transduction assessed rhBMP-2 production in huMSCs at 5 MOI (Fig. 1C). Non-transduced cells expressed BMP-2 below the detection threshold of the ELISA kit used and are not shown. Cells had low initial expression of BMP-2 which increased over time. BMP-2 mean expression was 0.488 pg BMP-2/cell 120 hours post-transduction but was highly variable between replicates, with a standard error of 0.358 pg/cell.
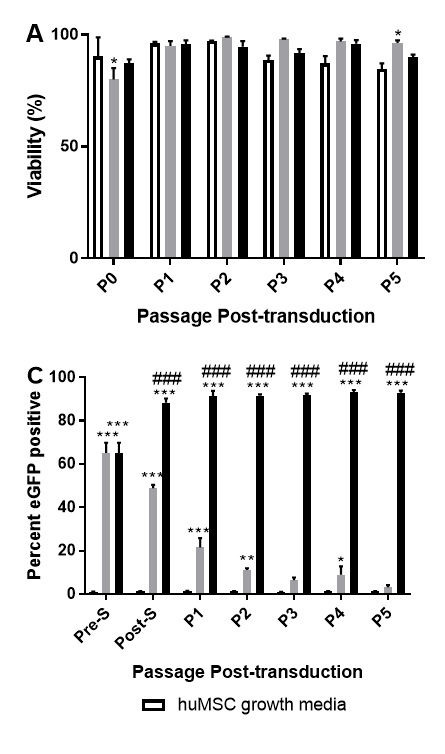
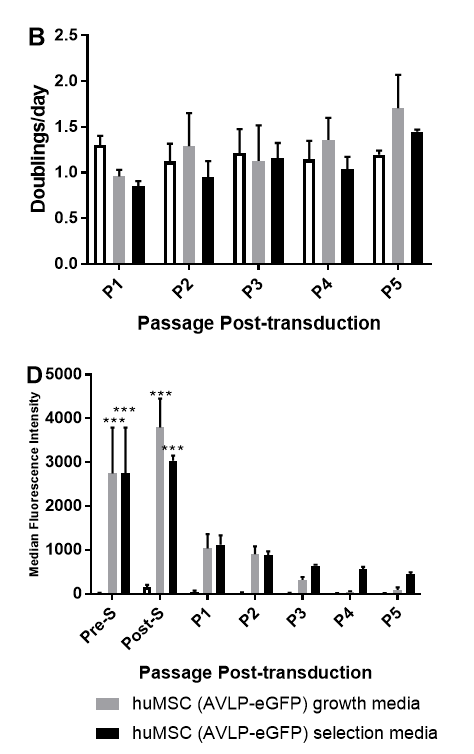
At each passage, the cells were counted, and their viability assessed with Trypan Blue (Fig. 3A). Viability of all groups was close to 100% across passages. There was a slight initial decrease in viability (p=0.0415) in the huMSC (AVLP-eGFP) group receiving complete medium after transduction compared to huMSCs, but this difference was not significant after one passage. Viability of huMSC (AVLP-eGFP) group that underwent hygromycin selection did not differ from that of the huMSCs. There were no significant differences between the growth rate of any of the groups as measured by doublings per day, with all groups maintaining a proliferation rate of approximately 1.0-1.5 doublings per day (Fig. 3B).
A portion of the MSCs harvested at each passage were used in flow cytometry analysis for eGFP expression. The initial transduction efficiency with AVLP-GFP was roughly 60%. Selection improved the percentage of GFP+ cells to near 90% across multiple passages, while subculturing cells in growth medium saw a steady decrease in the percentage of GFP+ cells to under 10% (Fig. 3C). However, when , when comparing the mean fluorescence intensity of GFP+ cells (Fig. 3D), a significant decrease from post-selection to P5 can be seen for huMSC (AVLP-eGFP) in both selection (p<0.0001) and growth media (p<0.0001).
Figure 3: AVLP-eGFP selection in huMSCs. Human umbilical mesenchymal stem cells (huMSCs) were transduced with AVLP-eGFP and selected for with hygromycin B while being sequentially expanded. The group undergoing selection retained similar viability (A) and proliferation (B) compared to transduced cells in growth medium and nontransduced cells. Furthermore, they maintained high expression of eGFP (C), while the median fluorescence intensity of eGFP in both transduced groups decreased greatly during culture (D). N=3, mean +/- SEM. Significant difference from “huMSC” is indicated by “*”, from “huMSC (AVLP-eGFP) growth medium” by “#”. Two-way ANOVA with Bonferroni’s test.
Viability of huMSC (AVLP-BMP2) ranged from 75% to 95% across three passages (Fig. 4A). The viability of the transduced cells in selection medium trended somewhat lower than that of the transduced cells in growth medium, but there were no significant differences between them. The proliferation rate of huMSC (AVLP-BMP2) was 1.02-1.24 doublings/day in growth medium and 0.96-1.20 doublings/day in selection medium (Fig. 4B). There were no significant differences between the proliferation rate of huMSC (AVLP-BMP2) in selection or growth medium. Hygromycin-B selection of cells transduced with AVLP-BMP2 did not alter BMP-2 production (Fig. 4C). There was a low level of BMP-2 secretion pre-selection, which was not improved post-selection and post-passaging, and levels varied throughout.
Discussion
The cost and morbidity associated with conventional bone grafts has led to interest in the development of osteogenic gene therapy. The two most-used viral systems to accomplish this are adenoviruses and lentiviruses, each with advantages and disadvantages [31]. Adenoviral vectors are highly efficient for short-term expression, but wane over the long term and may induce an immune response [17, 32]. This has been shown to interfere with bone formation [31, 33]. Conversely, lentiviruses permanently integrate into the host genome, but in doing so incur significant risk of insertional mutagenesis as seen in clinical trials [34, 35].
In this study, we investigated the use of AVLP as a vector for inducing expression of BMP-2 in mesenchymal stem cells due to its lack of cytopathic effect or immune response despite infecting many different cell types. Cells were transduced with AVLP to produce BMP-2 in quantities approaching 3 pg/cell at 96 hours post-transduction in some cases. Our group has previously attained BMP-2 expression of 0.7 pg/cell in adenvirus-transduced MSCs, and 1.5 pg/cell in lentivirus-transduced MSCs [21]. Adenovirus-transduced MSCs successfully induced heterotopic bone formation in rats, while the lentivirus-transduced MSCs successfully bridged a rat femur model of a critical-sized defect.
There have been several other attempts to create a stable line of MSCs expressing an exogenous protein using viral and non-viral vectors. For example, Sweeney et al successfully used a foamy virus vector to maintain high expression of GFP in MSCs over 10 passages as measured by both percent GFP positive cells and MFI [36]. However, as a retrovirus, the foamy virus will face safety concerns due to its integration into the host genome. In this study, AVLP induced exogenous gene expression in MSCs. Importantly, AVLP-transduced cells were selected for and propagated without loss of viability or growth potential, and huMSC (AVLP-eGFP) were maintained at high purity over multiple passages for at least 30 days. This duration of expression is consistent with previous experiments on AVLP-eGFP expression in epithelial cells, which maintained eGFP expression for 42 days without passaging and selection [29]. However, we were unable to create cells that consistently expressed high amounts of BMP-2 using AVLP-BMP2. This may be explained by the median fluorescence intensity (MFI) data of huMSC (AVLP-eGFP) (Fig. 3D). These cells survived selection and were approximately 90% positive for eGFP over several passages. However, the MFI of those same cells, roughly correlated with the amount of eGFP produced, drops steeply with passaging. This suggests that the level of expression or copy number of the AVLP-eGFP genome required for a cell to survive selection is quite low, which limits AVLP’s usefulness in synthetic bone grafts. In another study involving genetic engineering of MSCs, electroporation of a plasmid containing eGFP and neomycin phosphotransferase with human MSCs produced cells that, after selection, were highly positive for eGFP, even over many passages. However, similar to our findings, the eGFP MFI decreased over many passages in selection [37].
Figure 4: AVLP-BMP selection in huMSCs. Human umbilical mesenchymal stem cells (huMSCs) were transduced with AVLP-BMP and selected for with hygromycin B while being sequentially expanded. The group undergoing selection retained similar proliferation (A) and viability (B) but displayed high variability in expression of BMP-2 (C), while compared to transduced cells in growth medium. Non-transduced cells did not secrete detectable quantities of BMP-2 and are not shown. n=2, mean +/- SEM. Two-way ANOVA with Bonferroni’s test.
AVLP-eGFP was able to induce consistent, high, and sustained expression of eGFP in MSCs. There are several options for improving the ability of AVLP to consistently induce high BMP-2 expression. It may be possible to infect cells at a high MOI without a cytopathic effect. This is associated with an increased copy number per cell which could lead to increased protein expression [38]. Additionally, transfection aids such as Polybrene or protamine sulfate can be used to increase initial transduction efficiency by reducing charge repulsion between cells and the virus [39]. Different strategies for encouraging packaging, assembling, and budding of the construct may increase the efficiency and consistency of AVLP-BMP2. For example, the F protein, which mediates fusion with host cells, can be modified to increase its efficiency [40]. Finally, a different selection marker could be used to select for higher-expressing cells. The bleomycin analogue Zeocin™ has been shown to outperform hygromycin-B in selection efficiency [41]. In summary, there are numerous avenues for future work to enhance AVLP for use in gene therapy.
Additionally, AVLP could be useful in several applications beyond secretion of BMP-2. As mentioned above, AVLP has been used successfully in vaccines which induce expression of specific adhesion markers [29]. AVLP could be used for other cell surface modifications, including generation of CAR-T cells for cancer therapy, cell tracking, and labelling or modifying extracellular vesicles such as exosomes. Further work is needed to realize the full potential of the AVLP vector.
Article Info
Article Type
Research ArticlePublication history
Received: Wed 18, Jul 2018Accepted: Mon 06, Aug 2018
Published: Tue 28, Aug 2018
Copyright
© 2023 Steve Stice. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2018.02.007
Author Info
Biao He Christina Elling Huiling Wei Seth Andrews Steve Stice
Corresponding Author
Steve SticeRegenerative Bioscience Center, University of Georgia, 425 River Road, Athens GA 30602, USA
Figures & Tables





References
1. Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: An update. Injury 36: 20-27. [Crossref]
2. Toolan BC (2006) Current concepts review: Orthobiologics. Foot Ankle Int 27: 561-566. [Crossref]
3. Laurencin C, Khan Y, El-Amin SF (2006) Bone graft substitutes. Expert Review of Medical Devices 3: 49-57.
4. De Long WG Jr, Einhorn TA, Koval K, McKee M, Smith W (2007) Current concepts review: Bone grafts and bone graft substitutes in orthopaedic trauma surgery. J Bone Joint Surg Am 89: 649-658. [Crossref]
5. Campana V1, Milano G, Pagano E, Barba M, Cicione C, et al. (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25: 2445-2461. [Crossref]
6. Gómez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C, et al. (2015) Bone fracture healing: cell therapy in delayed unions and nonunions. Bone 70: 93-101. [Crossref]
7. McKay WF, Peckham SM, Badura JM (2007) A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE(®) Bone Graft). Int Orthop 31: 729-734. [Crossref]
8. Gothard D, Smith EL, Kanczler JM, Rashidi H, Qutachi O (2014) Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur Cell Mater 28: 166-208. [Crossref]
9. Govender S1, Csimma C, Genant HK, Valentin-Opran A, Amit Y, et al. (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84: 2123-2134. [Crossref]
10. Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, et al. (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J Bone Joint Surg Am 88: 1431-1441. [Crossref]
11. Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, et al. (2006) Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem 281: 23246-23253. [Crossref]
12. Zara JN, Siu RK, Zhang X, Shen J, Ngo R, et al. (2011) High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17: 1389-1399. [Crossref]
13. Tannoury CA, An HS (2014) Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14: 552-559. [Crossref]
14. Valdes MA, Thakur NA, Namdari S, Ciombor DM, Palumbo M (2009) Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Arch Orthop Trauma Surg 129: 1651-1657. [Crossref]
15. Obremskey WT, Marotta JS, Yaszemski MJ, Churchill LR, Boden SD, et al. (2007) Symposium. The introduction of biologics in orthopaedics: issues of cost, commercialism, and ethics. J Bone Joint Surg Am 89: 1641-1649. [Crossref]
16. Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, et al. (1999) The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81: 905-917. [Crossref]
17. Waehler R, Russell SJ, Curiel DT (2007) Engineering targeted viral vectors for gene therapy. Nat Rev Genet 8: 573-587. [Crossref]
18. Lin P, Correa D, Lin Y, Caplan AI (2011) Polybrene Inhibits Human Mesenchymal Stem Cell Proliferation during Lentiviral Transduction. PLoS One 6: e23891. [Crossref]
19. Milone MC, O’Doherty U (2018) Clinical use of lentiviral vectors. Leukemia. [Crossref]
20. Williams, D. A., & Thrasher, A. J. (2014) Concise Review: Lessons Learned From Clinical Trials of Gene Therapy in Monogenic Immunodeficiency Diseases. Stem Cells Transl Med 3: 636-642. [Crossref]
21. Mumaw J, Jordan ET, Sonnet C, Olabisi RM, Olmsted-Davis EA, et al. (2012) Rapid Heterotrophic Ossification with Cryopreserved Poly(ethylene glycol-) Microencapsulated BMP2-Expressing MSCs. Int J Biomater 2012: 861794. [Crossref]
22. Sonnet C, Simpson CL, Olabisi RM, Sullivan K, Lazard Z, et al. (2013) Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J Orthop Res 31: 1597-1604. [Crossref]
23. Yang Y, Zengel J, Sun M, Sleeman K, Timani KA, et al. (2015) Regulation of Viral RNA Synthesis by the V Protein of Parainfluenza Virus 5. J Virol 89: 11845-11857.
24. Hsiung G (1972) Parainfluenza-5 virus. Infection of man and animal. Prog Med Virol 14: 241-274. [Crossref]
25. McCandlish IA, Thompson H, Cornwell HJ, Wright NG (1978) A study of dogs with kennel cough. Vet Rec 102: 293-301. [Crossref]
26. Chen Z, Gupta T, Xu P, Phan S, Pickar A, et al. (2015) Efficacy of parainfluenza virus 5 (PIV5)-based tuberculosis vaccines in mice. Vaccine 33: 7217-7224. [Crossref]
27. Huang Y, Chen Z, Huang J, Fu Z, He B (2015) Parainfluenza virus 5 expressing the G protein of rabies virus protects mice after rabies virus infection. J Virol 89: 3427-3429. [Crossref]
28. Alaina J Mooney, Zhuo Li, Jon D Gabbard, Biao He, S Mark Tompkins (2013) Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J Virol 87: 354-362. [Crossref]
29. Wei H, Chen Z, Elson A, Li Z, Abraham M, et al. (2017) Developing a platform system for gene delivery: amplifying virus-like particles (AVLP) as an influenza vaccine. NPJ Vaccines 2: 32.
30. Kruse PF, Patterson MK (1973) Tissue culture: methods and applications: New York, Academic Press.
31. Balmayor ER, van Griensven M (2015) Gene therapy for bone engineering. Front Bioeng Biotechnol 3: 9. [Crossref]
32. Shayakhmetov DM (2010) Virus infection recognition and early innate responses to non-enveloped viral vectors. Viruses 2: 244-261. [Crossref]
33. Xu XL, Tang T, Dai K, Zhu Z, Guo XE, et al. (2005) Immune response and effect of adenovirus-mediated human BMP-2 gene transfer on the repair of segmental tibial bone defects in goats. Acta Orthop 76: 637-646. [Crossref]
34. Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, et al. (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302: 415-419. [Crossref]
35. Kohn DB, Candotti F (2009) Gene therapy fulfilling its promise. N Engl J Med 360: 518-521. [Crossref]
36. Sweeney NP, Regan C, Liu J, Galleu A, Dazzi F, et al. (2016) Rapid and Efficient Stable Gene Transfer to Mesenchymal Stromal Cells Using a Modified Foamy Virus Vector. Mol Ther 24: 1227-1236. [Crossref]
37. Peister A, Mellad JA, Wang M, Tucker HA, Prockop DJ (2004) Stable transfection of MSCs by electroporation. Gene Ther 11: 224-228. [Crossref]
38. Ellis EL, Delbruck M (1939) THE GROWTH OF BACTERIOPHAGE. J Gen Physiol 22: 365-384. [Crossref]
39. Lin P, Lin Y, Lennon DP, Correa D, Schluchter M, et al. (2012) Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl Med 1: 886-897. [Crossref]
40. Waning, D. L., Schmitt, A. P., Leser, G. P., & Lamb, R. A. (2002) Roles for the cytoplasmic tails of the fusion and hemagglutinin-neuraminidase proteins in budding of the paramyxovirus simian virus 5. J Virol 76: 9284-9297. [Crossref]
41. Lanza, A. M., Kim, D. S., & Alper, H. S. (2013) Evaluating the influence of selection markers on obtaining selected pools and stable cell lines in human cells. Biotechnol J 8: 811-821. [Crossref]
