Journals
Oncoplastic Management of a Giant Buschke Lowenstein Genital Tumor in a Nonimmunocompromised Patient
A B S T R A C T
Buschke-Löwenstein tumor (BLT) is a rare but impressive and aggressive entity and is mostly associated with Human Papilloma Virus (HPV) 6 or 11 infection in immunocompromised patients. It stands out because of the importance of the recurrences and the risk of progression to a malignant tumor. The first-line treatment remains local radical surgery. Other treatments are controversial, including radiotherapy, chemotherapy, and immunotherapy. We report a new case regarding the management of a giant BLT in a non-immunocompromised woman, treated in a three-staged approach, starting with a radical excisional surgery, followed by a delayed reconstruction and then radiotherapy.
Keywords
Bushke lowenstein, oncoplastic surger, vulvar carcinoma
Introduction
Buschke-Löwenstein tumor, also known as giant condyloma acuminatum, was first described in 1896, but was not considered as an entity on its own before 1925 when it was named by A. Buschke and L. Löwenstein [1]. This lesion consists of a pseudoepitheliomatous proliferation belonging to the verrucous carcinomas group. It is usually associated with a HPV 6 or 11 infection and occurs more frequently in middle-age men or immuno-compromised individuals [2]. This tumor is mostly located in the genital or peri-anal region. Typically, this pathology causes bleeding, pain and appears as a voluminous mass in a cauliflower-like shape [2]. Malign transformation has been reported up to 50% of cases [2]. Recurrence is frequent as well, reaching up to 65% of cases [3]. Metastases are rare [4]. The first line treatment is surgery, considering that alternatives have not yet been proven efficient [5].
In this case report, we highlight an unusual presentation of this tumor in an immunocompetent, elderly, female patient living in a developed country.
Case Presentation
A 69-year-old patient presented to an academic Belgian hospital for metrorrhagia that appeared the day before, recent pubalgia and persistent malodorous vaginal discharge for one year. In her medical history, she suffered from arterial hypertension treated with bisoprolol and perindopril, and from an auricular fibrillation treated by rivaroxaban. In her obstetrical history, she delivered four times vaginally, and stopped her gynecological follow-up after being menopaused at the age of 50. She is immunocompetent, and has been smoking for many years, but she stopped and switched for the electrical cigarette two years ago.
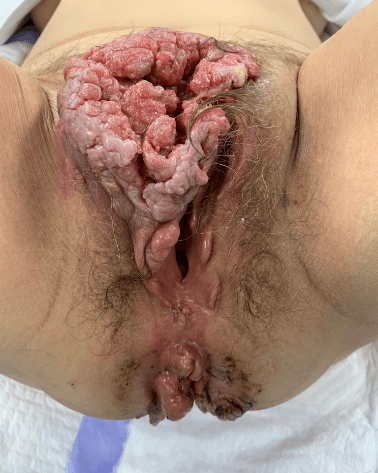
On physical examination the patient presented with a 10 centimeter in diameter bulging mass extending from the mons pubis to the superior part of the labia majora (Figure 1). Nodes were palpated in both inguinal regions. This mass was bleeding, malodorous and painful. The vagina, urethral meatus and uterine cervix were macroscopically healthy, whereas large peri-anal condylomas were observed. A bacterial smear displayed the presence of a multi-sensitive Proteus Mirabilis, as well as an Enterobacter Faecalis. A second sample revealed a multi-sensitive Pseudomonas Aeruginosa as well. The cervical smear and endovaginal sonogram were normal.
Figure 1: Giant Buschke Lowenstein lesion developed from the anterior part of the vulva (labia minor and Mons Venus). Condylomatous lesions are found also in the peri - anal region.
The blood test revealed an important anemia (Hb 6.2g/dL) for which she received two units of Red Blood Cells, and an inflammatory syndrome (CRP 22 mg/L, WBC 15790 /mm3, PLT 598000/mm3). Serologies for HIV, HBV, HCV, Syphilis and Chlamydia were negative. Punch biopsies were performed and the pathologic sample was compatible with a Buschke-Löwenstein tumor, even though a well differentiated invasive verrucous epidermal carcinoma could not be excluded. No high-risk HPV was found and none of the tested gene mutations were positive (TP53, BRAF, NOTCH1…).
An oncological staging was carried out through a pelvic MRI showing a 97x76x72 mm vulvar mass invading the right labium major and both labia minora. There was no extension to the perineum, whereas four right inguinal nodes (with a maximal dimension of 15 mm in diameter) and three left inguinal nodes (maximum of 13 mm) were identified. Due to mild hepatic enzyme abnormalities, an hepato-biliary ultrasound was performed showing a hyperechogenic focal lesion, compatible with a hepatic hemangioma as seen on the MRI. The brain tomodensitometry did not indicate any cerebral metastasis. A PET-CT showed nodes in the inguinal regions and right external iliac area, without any further extension, resulting in a cT1bN2bM0 stage tumor.
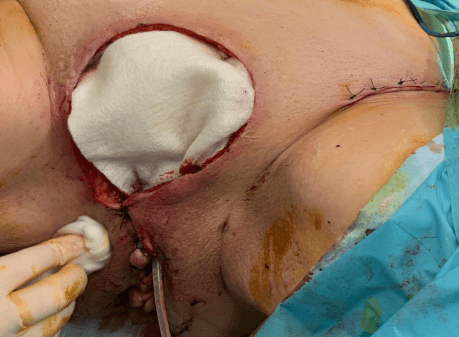
Figure 2: The inferior part of the resection (peri-urethral) is closed while the major part of the dissection is left opened for granulation and sterilization.
After a multidisciplinary oncological discussion, it was decided to perform a radical surgery, in collaboration with the plastic surgeons. The surgery consisted of a resection of the vulvar tumor and peri-anal condylomas, as well as a bilateral inguino-femoral lymphadenectomy. The inferior part of the wound was closed, whereas the major superior part was left opened in order to be reconstructed in a secondary procedure (Figure 2). During the post-operative stay, the local wound care consisted in wet-to-dry dressings, changed twice a day. This wound management was combined to an intravenous antibioprophylaxy (Cefazolin 2gr 3 times a day for seven days) led to an appropriate sterilization of the wound bed, with optimal granulation tissue.
Due to the removal of lymphatic nodes, the patient encountered a left inguinal lymphocele which was drained three times. The pathology reported a well differentiated invasive verrucous epidermal carcinoma, originating from a giant condyloma acuminatum/Buschke-Löwenstein tumor. The deep borders were clear, but the tumor was focally invading the lateral margins. The inguinal nodes were free of invasive cells, implying a pT1bN0M0 stage. The multidisciplinary tumor board recommended adjuvant irradiation after reconstruction in clear margins by the plastic surgery team.
Surgical reconstruction technique
Fifteen days after the first surgical excision, the patient was re-admitted in the operating theatre. All margins were resected to ensure disease-free margins. An abdominal pedicled advancement flap was performed, taking advantage of the abdominal skin excess of this 69-year-old patient, and reducing classical flaps donor sites morbidities for bilateral vulvar defects options (as DIEP flap or ALT flap). The lymphocele’s capsula was also entirely removed (Figure 3). The pathology of the lateral margins was free of tumor and the patient went home nine days after the reconstruction.
Figure 3: The edges of the wound are resected to ensure clear margins and an abdominal advancement flap closes the defect.
Discussion
Most of the literature on Buschke-Löwenstein Tumor (BLT) is composed of case reports, whereas only few studies were conducted without resulting in any clear guidelines. This pathology is a rare sexually transmitted disease, usually localized in the anogenital region. It is principally associated with an infection by papillomavirus [2, 6]. The tumor is mostly found in immunocompromised patients, suffering for example from HIV infection [2]. Surprisingly, our patient is immune-competent, thus standing out of the standards of a typical BLT presentation. Another factor frequently associated with the disease is smoking and our patient has a past history of smoking [2].
This kind of tumor is benign but locally aggressive, with a large capacity of infiltration and displacement of adjacent tissues [6-8]. Almost 50% of these tumors undergo malignant transformations, with an invasive squamous cell carcinoma component [2, 6]. The pathologic report after radical resection indicates a well-differentiated invasive squamous cell carcinoma of the wart type, originating from a BLT. Kridis et al. showed that the BLT differentiates into a squamous cell carcinoma by effraction of the basement membrane [8]. MRI and Pet-CT suspected loco-regional lymph node involvement. However, the histological results after resection were negative. Zhang and al., report four patients who also had lymph node resection during surgery, but none of them showed nodal metastasis [2]. Radical surgical resection of the tumor is the most common treatment and is associated with a good prognosis [2].
In this case, the multidisciplinary team decided to delay closure. This allowed to have the complete histological results of the tumor and its extension to the margins. Indeed, after surgical resection in our patient, the tumor was focally tangent to the lateral margins. In the study of Zhang and et al., 55,3% of the margins were positive after excision [2]. This is important to highlight because the delayed closure allowed the plastic surgeons to control the margins before closing the defect of tissue [2]. Furthermore, before any reconstruction, keeping the operating site wide opened allowed to sterilize it with daily local care. As described by Aviki et al. collaboration between gynaecologists and plastic surgeons for large vulvar defects improves tumor margin outcomes [9].
A simple reconstruction technique, covering the defect with well-vascularized tissue from the abdominal wall was performed. The patient could then go back to daily activities in a short postoperative period and receive the adjuvant radiotherapy. There is no consensus about other treatments, such as chemotherapy, radiotherapy and immunotherapy, neither if the treatment is better as neo-adjuvant or adjuvant [2]. In this case, the multidisciplinary team decided to add radiotherapy as adjuvant treatment because of the size of the tumor (10 cm), the invasion of the basal membrane (3 mm) and the increased size of the inguinal nodes.
The use of radiotherapy is controversial as verrucous condyloma may transform into poorly differentiated carcinoma and because it is also a risk factor for secondary dehiscence [4, 10]. For anorectal BLT, two case reports showed that neo-adjuvant therapy, respectively by chemo-radiation therapy or radiotherapy allowed a loco-regional control of the disease, thus avoiding colostomy [11, 12]. In the case report of Grodner and et al., the lesions were improved only by retroviral therapy in a HIV infected patient [13]. More studies are needed to better understand how to manage each case. The recurrence rate varied between 23,7% (median follow-up twenty-three months) and 66% [2, 6]. This probably depends on the time of follow-up. According the study of Chu and et al., the median time between surgery and the first recurrence is ten months [6].
Conclusion
BLT is a rare disease but locally destructive, that needs an optimal management. Surgical resection is considered as the gold standard and can include in our experience a delayed closure to ensure tumor free margins as well as a non-microbial contaminated wound before closing, sterilizing thus the operating site. Radiotherapy is one of the alternative treatments which was chosen as adjuvant therapy in our case. Effectiveness remains controversial in the literature, probably because of the lack of evidence-based guidelines. Collaboration between medical specialties and teamwork is essential. Larger studies are needed to determine which of the neoadjuvant or adjuvant treatments are the more efficient, and to study the long-term outcomes of the Buschke-Löwenstein Tumor.
Conflicts of Interest
None.
Abbreviations
ALT flap: anteroLateral Thight flap
Cm: Centimeters
CRP: C-Reactive Protein
CT-scan: Computed Tomography-scan
DIEP flap: Deep Inferior Epigastric perforating flap
Gr: Gram
Hb: Haemoglobin
HBV: Hepatitis B Virus
HCV: Hepatitis C Virus
HIV: Human Immunodeficiency Virus
Mm: Millimeters
MRI: Magnetic Resonance Imaging
PET-CT: Positron emission tomography–computed tomography
PLT: Platelets
WBC: White Blood Cells
Article Info
Article Type
Case ReportPublication history
Received: Mon 10, Feb 2020Accepted: Wed 26, Feb 2020
Published: Thu 12, Mar 2020
Copyright
© 2023 Philippe Simon. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.02.12
Author Info
M. Rimbault L. Paquier M. Tooulou N. Abboud Philippe Simon
Corresponding Author
Philippe SimonService de Gynécologie-Obstétrique, Belgium
Figures & Tables

References
- Buschke A, Lowenstein L (1925) Uber carcinomahnliche Condylomata Acuminata des Penis. Klin Wochenschr 4: 1726-1728.
- Zhang D, Gonzalez RS, Feely M, Umrau K, Lee H, et al. (2019) Clinicopathologic features of Buschke-Löwenstein tumor: a multi-institutional analysis of 38 cases. Virchows Arch. [Crossref]
- Creasman C, Haas PA, Fox TA Jr, Balazs M (1989) Malignant transformation of anorectal giant condyloma acuminatum (BuschkeLöwenstein tumor). Dis Colon Rectum 32: 481-487. [Crossref]
- Safi F, Bekdache O, Al-Salam S, Alashari M, Mazen T et al. (2013) Management of peri-anal giant condyloma acuminatum-A case report and literature review. Asian J Surg 36 : 43-52. [Crossref]
- Lévy A, Lebbe C (2006) Prise en charge des tumeurs de Buschke-Löwenstein. Ann Urol (Paris) 40: 175-178. [Crossref]
- Chu QD, Vezeridis MP, Libbey NP, Wanebo HJ (1994) Giant condyloma acuminatum (Buschke-Lowenstein tumor) of the anorectal and perianal regions. Dis Colon Rectum 37: 950-957. [Crossref]
- Sandoval I, Hernández R, Torres E, Yanque O (2019) Giant condylomata acuminata of Buschke-Lowenstein. J Obstet Gynaecol 1-2. [Crossref]
- Ben Kridis W, Werda I, Charfi S, Toumi N, Boudawara T, et al. (2019) Buschke-Lowenstein anal tumor: an ambiguous entity. Exp Oncol 41: 182-184. [Crossref]
- Aviki EM, Esselen KM, Barcia SM, Nucci MR, Horowitz NS, et al. (2015) Does plastic surgical consultation improve the outcome of patients undergoing radical vulvectomy for squamous cell carcinoma of the vulva? Gynecol Oncol 137: 60-65. [Crossref]
- Orkin BA (2013) Perineal reconstruction with local flaps: technique and results. Tech Coloproctol 17: 663-670. [Crossref]
- Indinnimeo M, Impagnatiello A, D’Ettorre G, Bernardi G, Moschella C et al. (2013) Buschke-Löwenstein tumor with squamous cell carcinoma treated with chemo-radiation therapy and local surgical excision: report of three cases. World J Surg Oncol 11: 231. [Crossref]
- Sobrado CW, Mester M, Nadalin W, Nahas SC, Bocchini SF, et al. (2000) Radiation-induced total regression of a highly recurrent giant perianal condyloma. Dis Colon Rectum 43: 257-260. [Crossref]
- Grodner C, Henn A, Lelièvre JD, Gallien S (2016) Successful improvement of Buschke-Löwenstein tumour in an HIV-infected patient with antiretroviral therapy alone. BMJ Case Rep 2016. [Crossref]
