Occurrence and Management of Empyema Thoracis During Covid-19 Times
Occurrence and Management of Empyema Thoracis During Covid-19 Times
A B S T R A C T
Introduction: Empyema is defined as an infected pleural fluid collection, evidenced either by purulent fluid or the presence of bacterial organisms. The aim of this study is to highlight the importance of early diagnosis and management of empyema in an attempt to avoid needless procedures and dreaded complications while shining a light into the warrant of a multidisciplinary approach that would had a paramount significance during the lockdown period in Greece, between March-June of 2020 due to COVID-19, in ameliorating those issues.
Patients and Methods: During the aforementioned period 12 patients were treated at our Department, 11 of them were male, ages ranging from 22-71 years old. The cause of empyema was parapneumonic effusion from a bacterial pneumonia in 10 patients and the other 2 were tuberculous empyema and extension of an intraabdominal process (Diffuse large B-cell lymphoma of the stomach). Predominately the patients were admitted to our Department with stage III empyema. Diagnosis was confirmed with CT scan and drainage of frank pus from the chest tube. All patients underwent chest tube insertion and antibiotic therapy nonetheless 9 of them required surgical management with VATS or open decortication.
Results and Conclusion: The impedance in seeking medical advice due to fear of COVID-19 and the insufficiency of an interdisciplinary approach in the management of those patients were determined as the reason for such high admittance with stage III empyema. The decision of open vs VATS decortication was made based on the medical status and history of each individual, the stage of the empyema and ultimately our ability to achieve the two primary goals of empyema treatment, complete evacuation, and lung re-expansion [1]. Eight patients underwent open decortication and drainage and one managed with VATS decortication. Postoperative complications were encountered in 3 patients which included prolonged air leak, surgical wound infection, and septic shock. One patient died from multiple organ failure due to postoperative septic shock. The duration of chest tube drainage varied from 5-15 days. The mean hospital stay was 13,5 days.
Keywords
Thoracic empyema, empyema management, Covid-19
Introduction
Empyema is defined as an infected pleural fluid collection, evidenced either by purulent fluid or the presence of bacterial organisms. Nowadays, nontuberculotic bacterial pneumonia is the leading cause of empyema with nearly 5% of the 1.2 million annual cases of pneumonia worldwide complicated with an empyema [2, 3]. Parapneumonic effusions from a bacterial infection are exudative and caused by inflammation of the pleura and lungs. The evolution from a simple parapneumonic effusion to a full-blown empyema occurs in 3 stages (Table 1) [4, 5]. Clinical presentation is not specific and depends on the patients’ immune status and virulence of the infectious agent. Commonly reported symptoms are shortness of breath, fever, cough, pleuritic chest pain and occasionally sputum. Empyema should be considered when a patient manifests these symptoms after a prolonged respiratory illness. Radiographic evaluation includes 1) CXR(F+P), bilateral decubitus films (free flowing /loculated empyema), assesses the completeness of drainage and lung expansion, 2) ultrasound to identify loculations/ pleural fibrosis, offers guidance for choosing site of thoracentesis and has the ability to differentiate between transudative and exudative effusions. 3) CT chest reveals the “split pleura” sign in nearly 70% of empyemas, identifies septations the total amount of pleural fluid, calculate the Hounsfield unit value, assesses the completeness of drainage and lung expansion [6-8].
Table 1: Stages of empyema.
|
Stage I (exudative stage) Uncomplicated parapneumonic effusion |
Stage II (fibrinopurulent stage) Complicated parapneumonic effusion |
Stage III |
|
Rapid free-flow of fluid caused by the movement of increased pulmonary interstitial fluid from the pleura into the pleura space. Pleura surfaces are inflamed and permeable |
Bacterial infection and fibrin deposition. Fluid color goes from clear yellow to frank pus. If stage II isn’t drained, the effusion organizes and evolves into stage III |
Fibroblastic ingrowth and collagen deposition. After 3-4 weeks a thickened membrane, “peel” develops that creates a trapped lung and ultimately restricts pulmonary function. Persistence of empyema cavity after 7-10 days of chest tube and failure of lung expansion |
|
Pleura fluid cultures are negative, pH> 7.2, LDH<1000 mg/100ml |
Cultures are positive, pH<7 glucose<40 |
|
|
Management requires antibiotics and drainage only if symptomatic |
Intrapleural fibrinolytic therapy with tPA and DNase can be used. Antibiotics plus chest tube drainage. |
VATS or open drainage, debridement and decortication. |
We report our experience with 12 patients during the lockdown period in Greece that spanned from March till June of 2020 in an attempt to highlight the importance of early and adequate intervention in order to avoid extensive procedures and dreaded complications.
Patients and Methods
During the aforementioned period 12 patients were treated at the Department of General Thoracic Surgery at the General Hospital of Attica K.A.T., Athens, Greece. The patients were predominately male (11 men and 1 woman) with ages ranging from 22 to 71. The cause of empyema was parapneumonic effusion from a bacterial pneumonia in 10 patients and the other 2 were tuberculous empyema and extension of an intraabdominal process (DLBCL of the stomach). 66,66 % of the patients developed right sided empyema.
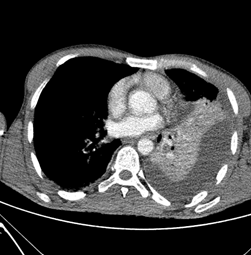
Figure 1: CT scan: Right sided pleural effusion with locules of gas and pneumothorax.
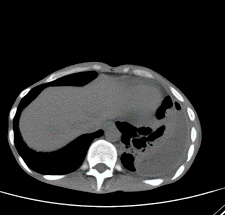
Figure 2: CT chest scan with IV contrast showing Left sided pleural effusion.
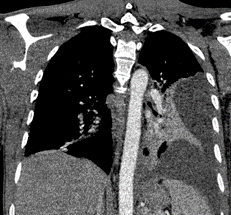
The similitude in all patients was the delay in seeking medical advice due to fear of Covid-19. Consequently, the majority of patients were admitted to our Department with a stage III empyema (9 pts from which 1 was presented with empyema necessitans) and the rest with stage II. Symptoms at the time of admission included shortness of breath (12 pts), fever (12 pts), productive cough (2pts) and pleuritic chest pain (12pts). Diagnosis was made by the means of CT and the drainage of frank pus from chest tube insertion. Chest tubes were inserted in all patients and samples were taken for culture, pH, glucose and LDH determination. Blood and sputum cultures were taken in all patients. All patients had characteristic radiological signs of empyema mentioned above (Figures 1-5).
Figure 3: CT scan coronal lung window: demonstrating left pleural effusion.
Figure 4: CT chest scan showing the characteristic “split pleura” sign.
Every single patient was started in broad spectrum empirical antibiotic treatment after chest tube insertion and cultures were taken. Afterwards the antibiotics were tailored to the culture results. Flexible bronchoscopy was performed in 1 patient due to atelectasis of the right upper and middle lobe. Follow up CT was performed in all patients to assess the adequacy of therapy and determine the need for further management. Surgical management with open decortication (8 pts) and VATS decortication (1 pt) was required for complete drainage, total peel removal and lung re-expansion. Postoperatively follow up with CT, CRP and pleural fluids cultures carried out in all patients. The duration of drainage was determined by normalization of cultures and CT scans.
Figure 5: CT chest scan showing left sided pleural effusion with septation and air locules.
Results
The microbiology report in all cases is summarized in (Table 2). In one patient prolonged air leak was present after open decortication and infusion of FFP through the chest tube was carried out four times as a feasible option to stop the air leak. Eventually this patient required a second thoracotomy, and a bronchopleural fistula was identified along with five point of leakage that were sutured with PTFE pledgets. Another patient experienced postoperative surgical wound infection with candida and pantoea and surgical debridement was applied alongside prolonged antibiotic therapy. In all cases the surgery was performed with a double lumen endobronchial tube under general anaesthesia. The duration of chest tube drainage varied from 5-15 days. CT scan was performed before discharge in all patients and displayed no pleural effusion and fully expanded lung. The mean hospital stay was 13,5 days. One patient experienced postoperative septic shock and died from multiple organ failure in ICU. No intraoperative mortality occurred (Table 3).
Table 2: The microbiology report.
|
Case |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Blood cultures |
- |
- |
- |
Staphylococcus aureus |
Staphylococcus hominis |
- |
- |
- |
- |
Acinetobacter baumani, Klebsiella pneumoniae |
Staphylococcus hominis |
Acinetobacter baumani, Candida kruseia |
|
Sputum cultures
|
- |
Stenotrophomonas maltophilia, Candida spp. |
- |
Candida spp. |
Acinetobacter baumani |
- |
- |
- |
- |
- |
Candida , Klebsiella pneumoniae |
- |
|
Pleura effusion cultures |
Streptococcus pneumoniae |
Streptococcus pneumoniae, Pseudomonas putida |
Fusobacterium nucleatum |
Staphylococcus aureus |
Acinetobacter baumani |
Streptococcus pneumoniae |
Strep. intermedius |
Staphylococcus aureus |
Klebsiella pneumoniae, Acinetobacter baumani |
Acinetobacter baumani, Klebsiella pneumoniae |
Candida |
Acinetobacter baumani, Candida kruseia |
|
Intraoperative pus cultures |
- |
- |
- |
Staphylococcus aureus |
Mycobacterium tuberculosis |
- |
Candida albicans |
- |
- |
Acinetobacter baumani, Klebsiella pneumoniae |
Staphylococcus hominis, Candida, Klebsiella pneumoniae, Enterobacter |
Acinetobacter baumani, Candida kruseia |
Table 3: Summary of 12 patients with thoracic empyema.
|
Patient |
Age (years) |
Sex |
Cause |
Treatment |
Complications |
Hospital stay (days) |
Outcome |
|
1 |
35 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
6 |
Discharged |
|
2 |
30 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
8 |
Discharged |
|
3 |
61 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
7 |
Discharged |
|
4 |
22 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
12 |
Discharged |
|
5 |
26 |
M |
Tuberculosis |
Bronchoscopy and right thoracotomy for drainage open decortication and closure of bronchopleural fistula, antibiotics |
none |
14 |
Discharged |
|
6 |
58 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
11 |
Discharged |
|
7 |
54 |
M |
Bacterial pneumonia |
Left thoracotomy with open drainage, decortication and drainage of a left upper lobe abscess, antibiotics |
Prolonged air leakage, 4 times infusion of FFP through the chest tube was performed but the air leakage continued so a 2nd operation was needed to suture 5 points of leakage and close the bronchopleural fistula that had created |
17 |
Discharged |
|
8 |
42 |
M |
Bacterial pneumonia |
Left VATS with drainage and decortication, antibiotics |
none |
9 |
Discharged |
|
9 |
71 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
11 |
Discharged |
|
10 |
45 |
F |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
Septic shock |
25 |
Died |
|
11 |
48 |
M |
Bacterial pneumonia Empyema necessitans |
Right thoracotomy with open drainage and decortication, antibiotics |
Postoperative surgical wound infection |
22 |
Discharged |
|
12 |
60 |
M |
DLBCL of stomach Extension of an abdominal process |
Left thoracotomy with open drainage and decortication, antibiotics |
none |
20 |
Discharged |
Discussion
Empyema is associated with elevated morbidity and mortality, around 20% to 30% of patients affected will either die or required further surgery in the first year after developing empyema, putting early and thorough intervention at the epicenter of each empyema case [9]. Contingent upon the origins of the infection, is it community-acquired or hospital-acquired, the bacteriology of empyema may change. Also, comorbidities of the patients need to be taken into consideration. For community-acquired empyema, gram-positive bacteria are more common, especially Streptococcus species. In this setting, the presence of gram-negative bacteria has been associated with increased comorbidities of patients with alcohol abuse, gastroesophageal reflux disease (GERD), and diabetes. In hospital-acquired empyema, Staphylococcus aureus, (methicillin-resistant S. aureus (MRSA)) and Pseudomonas are more common. Some risk factors have been identified as unique for developing empyema. Among these factors are diabetes mellitus, intravenous drug abuse, immunosuppression, gastric acid reflux, and alcohol abuse [10].
A scoring system has been developed to assess mortality in 3 months upon presentation of patients with empyema. This system is called the RAPID score. The parameters included in the system are kidney function (renal), age, presence, or albescence of pus, hospital-acquired versus community-acquired infection, and albumin levels (diet). Patients with a score of more than 5 had poor outcomes [11].
Once the decision has been made to proceed with operative intervention, the choice of open versus thoracoscopic approach must be made with two primary goals in mind: 1) complete evacuation of potentially infected fluid and/or material and 2) complete re-expansion of the lung. Specific benefits that have been demonstrated when comparing VATS treatment of acute empyema versus thoracotomy include improved postoperative pain control, shorter length of stay, less blood loss, less respiratory compromise, and reduction in postoperative complications including 30 day mortality. One of the major decision points during any attempted VATS procedure is when to convert to an open thoracotomy. Uncontrollable bleeding, injury to structures not amenable to thoracoscopic repair, and acute intolerance of single- lung ventilation are universal indications for immediate conversion to thoracotomy. With respect to empyema, two additional factors that should prompt consideration for conversion are lack of surgical progression and failure to ultimately achieve the two goals of empyema therapy (evacuation and expansion) [12].
In conclusion, managing thoracic empyema during COVID-19 times is extremely challenging and requires the alertness of an interdisciplinary team with a primary pursue of an early and adequate treatment in order to avoid unnecessary and extensive interventions.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 26, Oct 2020Accepted: Fri 06, Nov 2020
Published: Thu 19, Nov 2020
Copyright
© 2023 Vakouftsi Alexia- Christina. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.11.12
Author Info
Vakouftsi Alexia- Christina Michos Thrasyvoulos Stanitsa Nikoleta Stergiou Dimitrios Mauris Loukas Roumpaki Anastasia Stamatelopoulos Athanasios Chantziantoniou Christos Gakidis Ioannis Michos Petros
Corresponding Author
Vakouftsi Alexia- ChristinaGeneral Thoracic Department, of General Hospital of Attica “KAT”, Athens, Greece
Figures & Tables
Table 1: Stages of empyema.
|
Stage I (exudative stage) Uncomplicated parapneumonic effusion |
Stage II (fibrinopurulent stage) Complicated parapneumonic effusion |
Stage III |
|
Rapid free-flow of fluid caused by the movement of increased pulmonary interstitial fluid from the pleura into the pleura space. Pleura surfaces are inflamed and permeable |
Bacterial infection and fibrin deposition. Fluid color goes from clear yellow to frank pus. If stage II isn’t drained, the effusion organizes and evolves into stage III |
Fibroblastic ingrowth and collagen deposition. After 3-4 weeks a thickened membrane, “peel” develops that creates a trapped lung and ultimately restricts pulmonary function. Persistence of empyema cavity after 7-10 days of chest tube and failure of lung expansion |
|
Pleura fluid cultures are negative, pH> 7.2, LDH<1000 mg/100ml |
Cultures are positive, pH<7 glucose<40 |
|
|
Management requires antibiotics and drainage only if symptomatic |
Intrapleural fibrinolytic therapy with tPA and DNase can be used. Antibiotics plus chest tube drainage. |
VATS or open drainage, debridement and decortication. |
Table 2: The microbiology report.
|
Case |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
Blood cultures |
- |
- |
- |
Staphylococcus aureus |
Staphylococcus hominis |
- |
- |
- |
- |
Acinetobacter baumani, Klebsiella pneumoniae |
Staphylococcus hominis |
Acinetobacter baumani, Candida kruseia |
|
Sputum cultures
|
- |
Stenotrophomonas maltophilia, Candida spp. |
- |
Candida spp. |
Acinetobacter baumani |
- |
- |
- |
- |
- |
Candida , Klebsiella pneumoniae |
- |
|
Pleura effusion cultures |
Streptococcus pneumoniae |
Streptococcus pneumoniae, Pseudomonas putida |
Fusobacterium nucleatum |
Staphylococcus aureus |
Acinetobacter baumani |
Streptococcus pneumoniae |
Strep. intermedius |
Staphylococcus aureus |
Klebsiella pneumoniae, Acinetobacter baumani |
Acinetobacter baumani, Klebsiella pneumoniae |
Candida |
Acinetobacter baumani, Candida kruseia |
|
Intraoperative pus cultures |
- |
- |
- |
Staphylococcus aureus |
Mycobacterium tuberculosis |
- |
Candida albicans |
- |
- |
Acinetobacter baumani, Klebsiella pneumoniae |
Staphylococcus hominis, Candida, Klebsiella pneumoniae, Enterobacter |
Acinetobacter baumani, Candida kruseia |
Table 3: Summary of 12 patients with thoracic empyema.
|
Patient |
Age (years) |
Sex |
Cause |
Treatment |
Complications |
Hospital stay (days) |
Outcome |
|
1 |
35 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
6 |
Discharged |
|
2 |
30 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
8 |
Discharged |
|
3 |
61 |
M |
Bacterial pneumonia |
Chest tube drainage And antibiotics |
none |
7 |
Discharged |
|
4 |
22 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
12 |
Discharged |
|
5 |
26 |
M |
Tuberculosis |
Bronchoscopy and right thoracotomy for drainage open decortication and closure of bronchopleural fistula, antibiotics |
none |
14 |
Discharged |
|
6 |
58 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
11 |
Discharged |
|
7 |
54 |
M |
Bacterial pneumonia |
Left thoracotomy with open drainage, decortication and drainage of a left upper lobe abscess, antibiotics |
Prolonged air leakage, 4 times infusion of FFP through the chest tube was performed but the air leakage continued so a 2nd operation was needed to suture 5 points of leakage and close the bronchopleural fistula that had created |
17 |
Discharged |
|
8 |
42 |
M |
Bacterial pneumonia |
Left VATS with drainage and decortication, antibiotics |
none |
9 |
Discharged |
|
9 |
71 |
M |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
none |
11 |
Discharged |
|
10 |
45 |
F |
Bacterial pneumonia |
Right thoracotomy with open drainage and decortication, antibiotics |
Septic shock |
25 |
Died |
|
11 |
48 |
M |
Bacterial pneumonia Empyema necessitans |
Right thoracotomy with open drainage and decortication, antibiotics |
Postoperative surgical wound infection |
22 |
Discharged |
|
12 |
60 |
M |
DLBCL of stomach Extension of an abdominal process |
Left thoracotomy with open drainage and decortication, antibiotics |
none |
20 |
Discharged |


References
- Bostock IC, Sheikh F, Millington TM, Finley DJ, Phillips JD (2018) Contemporary outcomes of surgical management of complex thoracic infections. J Thorac Dis 10: 5421-5427. [Crossref]
- Carboni GL, Fahrner R, Gazdhar A, Printzen G, Schmid RA et al. (2008) Comparison of procalcitonin and CrP in the postoperative course after lung decortication. Eur J Cardiothorac Surg 33: 777-780. [Crossref]
- Cheng YJ , Wu HH, Chou SH , Kao EL (2002) Video-assisted thoracoscopic surgery in the treatment of chronic empyema thoracis. Surg Today 32: 19-25. [Crossref]
- Light RW (2006) Parapneumonic effusions and empyema. Proc Am Thorac Soc 3: 75-80. [Crossref]
- Light RW, Girard WM, Jenkinson SG, George RB (1980) Parapneumonic effusions. Am J Med 69: 507-512. [Crossref]
- Schachter EN, Kreisman H , Putman C (1976) Diagnostic problems in suppurative lung disease. Arch Intern Med 136: 167-171 . [Crossref]
- Sajadieh H, Afzali F, Sajadieh V, Sajadieh A (2004) Ultrasound as an alternative aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci 1019: 585-592 . [Crossref]
- Stark DD, Federle MP, Goodman PC, Podrasky AE, Webb WR et al. (1983) Differentiating lung abscess and empyema: radiography and computed tomography. AJR Am J Roentgenol 141: 163-167. [Crossref]
- Roozendaal LMV, Gool MHV, Sprooten RTM, Maesen BAE, Poeze M et al. (2018) Surgical treatment of bronchial rupture in blunt chest trauma: a review of literature. J Thorac Dis 10: 5576-5583. [Crossref]
- Garvia V, Paul M (2020) Empyema. In: StatPearls. [Crossref]
- White HD, Henry C, Stock EM, Arroliga AC, Ghamande S (2015) Predicting Long-Term Outcomes in Pleural Infections. RAPID Score for Risk Stratification. Ann Am Thorac Soc 12: 1310-1316. [Crossref]
- Shen KR, Bribriesco A, Crabtree T, Denlinger C, Eby J et al. (2017) The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 153: e129-e146. [Crossref]
