“Non-Parasitic” and “Non-Iatrogenic” Chyluria: Its Diagnosis and Treatment
A B S T R A C T
Chyluria occurs in all its forms with milky urine, recurrent episodes of acute urinary retention, left renal colic and proteinuria. In non-parasitic or iatrogenic diseases, it is secondary to communicate between the cisterna system of the chyli and the lymphatics of the calyx system of the left urinary tract with a retrograde passage of a kilo and its appearance in the urine which therefore take on a milky appearance [1, 2]. Sometimes, episodically, especially after the ingestion of a high-fat meal, the quantity of kilo is so abundant that it can cause obstruction of the upper urinary tract and of the bladder, resulting in renal colic or acute urinary retention that may require their unblocking with double J stent or bladder catheter [3, 4]. After conservative attempts with a fat-free diet or with the parenteral diet, in case of their failure, surgery must be performed by performing a para-aortic and renal hilum lymphadenectomy and, in severe cases, with intraperitonealization of the kidney and left ureter. This is the case of the patient reported below and successfully treated recently with an innovative “open” surgical technique.
Keywords
Chyluria, milky urine, para-aortic lymphadenectomy, renal and ureteral intraperitonealization
Case Presentation
A 62-year-old patient who weighs 76 kg and is 176 cm tall. The physiological anamnesis shows that he was born from eutocic birth, with physiological development, normal appetite, normal alvo. He drinks a glass of wine a day and is an ex-smoker of 20 cig/day. In his family history, he reports that he has two children alive, without disease, father who died of bowel cancer at age 89 and mother who died of liver cirrhosis at age 83. In his medical history, he reports episodes of gastroesophageal reflux and prostatic hypertrophy with cervico-urethral obstruction. The patient denies traveling to endemic places for parasitic infections. Remote surgical history: right inguinal hernioplasty (aged 9 years); left inguinal hernioplasty (at the age of 54); right crural hernioplasty (at the age of 58); hemorrhoidectomy (in October 2017). Of high etiopathogenetic interest, it can be a high ligature of left varicocele at the age of 32! On physical examination, it does not show any significant alterations: good general conditions, rhythmic pulse, eupnoeic breath, painless and painful abdomen, palpable masses are not appreciated, and the sign of Giordano is bilateral negative. On admission, he performed blood chemistry tests: blood count, renal function indices, electrolytes, liver function indices, albumin and total proteins were in the normal range.
The patient's history is traced back to October 2017, when he started to present whitish urine output [1, 2]. In December 2017, he had the first episode of acute urinary retention (RUA), for which the bladder catheter was positioned with the consequent emission of whitish urine [3, 4]. For this reason, he was hospitalized in the Infectious Diseases department, where UTI was excluded: Negative urine culture; negative urine culture for Neisseria; negative real-time PCR for Chlamydia trachomatis on urine; negative Schistosoma research; negative Multiplex PCR for Mycoplasma. During this hospitalization, protein immunofixation was also performed in the urine/Bence Jones which showed negative result. Immunofixation of whey proteins showed the presence of a small monoclonal component IgA lambda and protein electrophoresis in urine showed non-selective glomerular proteinuria (proteinuria <1000 mg / dl) [4].
In January 2018, he was hospitalized in the U.O. of Nephrology of the San Giovanni Rotondo hospital, where he performed lymphoscintigraphy (10/01/2018): “probable lymphatic extravasation along the likely left ureteral course at the level of L4-L5” [5, 6]. During this hospital stay, he was screened for HBV, HCV, HIV and for autoimmunity (anti-dsDNA, ANA, AMA, ASMA, APCA, FR) with a negative response. He performed CT abdomen with contrast that did not show any spillage of contrast from the urinary tract. He performed parenteral nutrition for about 3 weeks, resulting in regression of chyluria, but when he resumed the oral diet, despite an alipidic diet, he had an immediate resumption of chyluria [7]. In February 2018, he was hospitalized in the Hematology department of the San Giovanni Rotondo hospital, where bone marrow aspiration and bone biopsy were performed without pathological results. During the hospitalization, he also performed pelvis and spinal MRI with not so clinically relevant results. In March 2018, he performed total-body PET / CT with 18F-FDG which showed no other abnormalities, except for a focal area of increased metabolic activity in correspondence with a hypodense-like nodular formation, located in the context of the left lobe of the thyroid gland. In April 2018, he performed cystoscopy: “prostate adenoma projecting into the bladder, rigid neck, normo-ejaculating meatus”.
In the meantime, episodes of RUA were repeatedly associated with the emission of urine, heavily loaded with a gelatinous kilo which caused the obstruction [1-4]. For this reason, from 09/03/2019 he was a permanent bladder catheter carrier and was forced to change it periodically or in case of obstruction. On 11/03/2019, he performed abdominal-thorax-head CT with contrast following a hyperlipidic diet requested by us: “... the calico-pyelic-ureteral cavities do not appear dilated, do not have thickened walls, nor extra-luminous extravasation of contrast. In the left para-aortic retroperitoneal area, it seems to recognize a slight ectasia of some lymphatic vessels which, however, do not appear to contract close contiguous relationships with the ipsilateral ureter. There is slight imbibition of the adipose tissue of the vascular space of the retroperitoneum ...”; other findings are of no relevance.
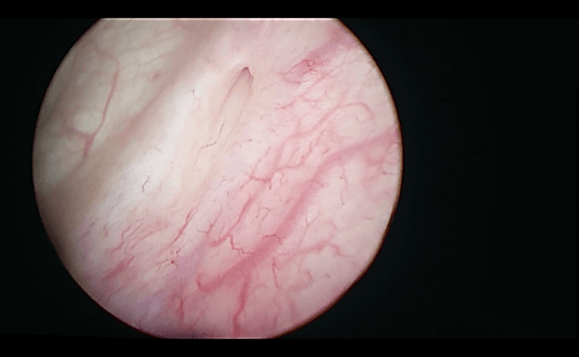
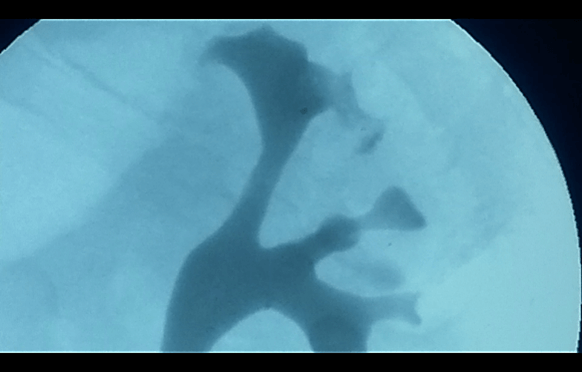
On 12/03/2019, he was hospitalized in our department of Urology I of the Polyclinic of Bari to exclude alterations affecting the lower urinary tract and, above all, to confirm the side of the kilo emission. During this hospitalization, urethrocystoscopy was performed with a prolonged observation of the emission of urine from the meatus. This, therefore, excluded alterations affecting the anterior and posterior urethra, highlighting only one detected bladder neck, and as expected, confirmed the escape of milky urine from the left ureteral meatus (Figure 1). Therefore, retrograde pyelography was performed which showed minimal spreading of contrast starting from the upper left calyx (Figure 2) [8]. JJ stent 6 Fr x 28 cm under the fluoroscopic guide and bladder catheter (foley 18 ch) were positioned as a further attempt to “conservatively” resolve the chyluria.
Figure 1: Milky urine from the left ureteral meatus.
Figure 2: Retrograde pyelography.
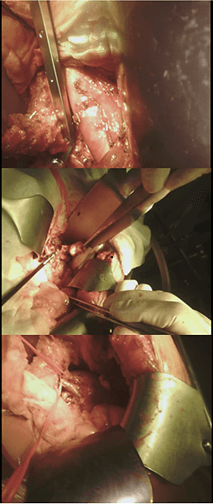
Figure 3: Para-aortic lymphadenectomy and renal and ureteral intraperitonealization.
From the day of the operation, in an attempt to keep the high urinary tract under suction and allow it to close, assuming a communication between the upper calves fornix and the cisterna chyli, the patient has undergone parenteral nutrition for 10 days, with the immediate disappearance of the chyluria [7]. After 10 days of fasting, the patient resumed the diet orally, with a high intake of fatty foods. About 48 hours after resuming oral nutrition, the patient experienced the escape of milky urine from the bladder catheter. The patient was therefore discharged and then hospitalized again at our department to undergo surgery. On 09/04/2019, an “open” lymphadenectomy plus kidney and left ureter intraperitonealization was then performed, described as follows: “Xipho-pubic incision. Isolation of aorta from the emergence of the ipsilateral renal artery up to the lower mesenteric artery. Isolation of the left kidney and ipsilateral ureter. Para-aortic lymphatic tissue and left renal ile of marked chronic inflammatory appearance are removed. The removal of fat from the left renal lodge with complete exposure of the ipsilateral kidney. The left ureter is completely freed from the paraureteral tissue with aspects of chronic inflammation. Intraperitonealization of the left kidney (it is wrapped in the omentum flap) and of the ipsilateral ureter” (Figure 3).
The patient, already during the hospital stay in our ward, resumed free diet without the appearance of chyluria. On 09/08/2019, he performed abdomen CT with contrast that did not show any spreading and regular excretion of urine from the excretory tract. Currently, about 1 year after the surgery, the patient, despite a free and high-fat diet, has not shown the reappearance of chyluria.
Discussion
In literature, there are few cases of non-parasitic, non-iatrogenic chyluria, the cause of which is not easily identifiable, despite the multiple diagnostic means used and which occurs with recurrent episodes of acute urinary retention and in the absence of renal colic or hydronephrosis. We wanted to describe this case by highlighting all the steps as describing a diagnostic-therapeutic path because, in fact, there is nothing in the literature, since it is a very rare case: an “idiopathic chyluria”, resistant to all the attempts described in the literature of "best conservative treatment" such as alipidic parenteral feeding and continuous drainage of the upper and lower urinary tract and resolved with “radical” surgery of the retroperitoneum. The extensive para-aortic, retroaortic and entire interaortocaval lymphadenectomy, intraperitonealization of the kidney and ureter and their complete covering with a large, vascularized flap of omentum has brought together the three isolated ways described in the literature to solve cases of chyluria even with laparoscopic or robotic minimally invasive techniques [9, 10]. We wanted to resort to this complex “radical” surgery “open” technique for the severity of the case described, for the long duration of the pathology and to reduce to a minimum the recurrence that weighs, even if in a low percentage, on the other techniques adopted in isolation. The use of this “complete” successful treatment technique of “non-iatrogenic and non-parasitic chyluria” can, in the manner described in this case, represent a model of diagnosis and treatment of particularly complex cases.
Article Info
Article Type
Case ReportPublication history
Received: Tue 23, Feb 2021Accepted: Tue 23, Mar 2021
Published: Tue 04, May 2021
Copyright
© 2023 Pier Paolo Prontera. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2021.03.20
Author Info
Marco Rinaldi Pier Paolo Prontera Francesco Saverio Grossi Marco Spilotros Giuseppe Lucarelli Gaetano De Rienzo Pasquale Ditonno Michele Battaglia
Corresponding Author
Pier Paolo PronteraMedical Director in Urology, S.S. Annunziata Hospital, Taranto, Italy
Figures & Tables
References
1.
Guttila A, Beltrami P, Bettin L, Galantini A, Dal Moro F
et al. (2017) Chyluria: the state of the
art. Urologia 84: 65-70. [Crossref]
2.
Stainer V, Jones
P, Juliebø SO, Beck R, Hawary A (2020) Chyluria: what does the clinician need
to know? Ther Adv Urol 12: 1756287220940899. [Crossref]
3.
Taylor TV,
Strachan AW, Isherwood I, Moore T (1975) Non-parasitic chyluria (presenting
with urinary retention). Br J Urol 47: 419-423. [Crossref]
4.
Poitou C, Kheder
Elfekih R, Djebbar M, Perreira P, Deray G et al. (2009) [Chyluria presenting as
milky urine and nephrotic-range proteinuria and milky urine: chyluria or
glomerulopathy? Case report and literature review]. Nephrol Ther 5:
642-647. [Crossref]
5.
Yuan Z, Luo Q,
Chen L, Luo Q, Zhu R (2010) The role of radionuclide lymphoscintigraphy in
chyluria. Hell J Nucl Med 13: 238-240. [Crossref]
6.
Pui MH, Yueh TC
(1998) Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. J
Nucl Med 39: 1292-1296. [Crossref]
7.
Cortés S,
Rodríguez A, Gámez G (1978) [Chyluria]. Arch Esp Urol 31: 283-298.
[Crossref]
8.
Bally S, Demey A,
Arrivé L, Rullier C (2016) [Chyluria with "nephrotic syndrome-like"
presentation: Diagnostic and therapeutic approach]. Prog Urol 26:
1153-1156. [Crossref]
9.
Liu B, Zhang J, Li J, Li P, Han Z et al. (2014) Modified retroperitoneoscopic renal pedicle
lymphatic disconnection for intractable chyluria. Urology 83: 1195-1198.
[Crossref]
10. Barman N, Palese M (2016) Robotic surgery for
treatment of chyluria. J Robot Surg 10: 1-4. [Crossref]
