Multiple Recurrent Pneumothoraces and Thoracic Drain Insertion in a Mechanically Ventilated Patient Suffering from Methadone Induced Cardiomyopathy
A B S T R A C T
Objective: To describe the experience of a multimodal therapeutic approach in a patient with methadone-induced dilated cardiomyopathy who developed recurrent bilateral tension pneumothorax.
Setting: Department of Intensive Care.
Patient: A patient with methadone-induced cardiomyopathy and severe left ventricular dysfunction who after mechanical ventilation underwent bilateral tension pneumothorax and prolonged cardiovascular resuscitation (CPR).
Interventions: Cardiac Angiography, Multiple counter–shock (defibrillator dose), Multiple Thoracic Drains.
Case Report: A 56-year-old man with past IV drug abuse and severe left ventricular dysfunction was transferred from the intensive cardiac care unit (ICCU) to our intensive care unit (ICU) ward due to suspected aspiration pneumonia. Multiple attempts of weaning off mechanical ventilation were unsuccessful, followed by development of septic shock. Following cardiothoracic consultation, two thoracic drains were placed. Due to repeated events of bilateral tension pneumothorax and CPR attempts, a total of seven thoracic drains were placed, permitting rapid control and improvement in the patient status. The possibility of Extracorporeal Membrane Oxygenation (ECMO) was not considered as supportive care due to methadone use and severe secondary cardiomyopathy. In the following days, control and stabilization of the patient status was obtained. Vasopressor treatment withdrawal, cessation of drainage and removal of five thoracic access points were successfully performed prior to percutaneous tracheostomy. The two remaining drains were removed later on during hospitalization. After 29 days in the ICU, the patient was discharged to a step down ward.
Keywords
Methadone induced cardiomyopathy, severe left ventricular dysfunction, CPR, recurrent bilateral tension pneumothorax
Introduction
Methadone induced cardiomyopathy is well described in the literature [1]. Prolonged QT interval, QT dispersion, torsade de pointes (TdP) and life-threatening arrhythmias were all described in patients receiving methadone. Also depicted as secondary to methadone withdrawal was stress induced cardiomyopathy (Takotsubo syndrome) when receiving doses higher than 120 mg/day [2]. One of the challenges of an ICU physician today is obtaining control over multi-organ failure, such as septic shock combined with severe LV dysfunction followed by cardiogenic shock.
To our knowledge, multiple recurrent tension pneumothoraces with insertion of 7 thoracic drains, consecutive to CPR and mechanical ventilation, is rare in the literature. We describe here our experience managing a patient with methadone-induced cardiomyopathy with severe heart failure and multiple thoracic drains insertion following recurrent pneumothoraces.
Case Presentation
A 56-year-old patient with a history of intravenous drug abuse, hepatitis C virus carrier, diabetes mellitus and high dose methadone treatment was transferred to Hasharon Emergency Department (ED) after an event of severe arrhythmia at home described by the Emergency Medical Service as ventricular tachycardia with a pulse.
In the ED, an electrocardiogram (ECG) showed wide complex tachycardia with hemodynamic and respiratory failure. The patient received multiple synchronized shock doses after orotracheal intubation. Chest x-ray revealed bilateral pulmonary congestion.
The patient was put on intravenous amiodarone maintenance and transferred to the ICU with the intention of performing an emergency coronary angiography. Upon revealing normal coronary arteries, conservative medical management was initiated. Laboratory testing showed elevated troponin levels (221 ng/l) and a Transthoracic Echocardiogram (TTE) was performed revealing a Visually Estimated Ejection Fraction (LVEF) of 20% and normal right ventricular function (RVF).
In addition to severe cardiac failure, the patient suffered from aspiration pneumonia. When transferred to our department, he was hemodynamically stable but still heavily sedated via fentanyl 500 mcg/hour and midazolam 30 mg/hour. While waiting for the results of blood and sputum cultures, empiric antibiotic treatment with ceftriaxone-metronidazole was initiated.
On arrival, arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2) ratio revealed another deterioration (drop to 80 mmHg), therefore treatment with nitric oxide (NO) was initiated. A second TTE was preformed revealing another cardiac deterioration with LVEF of 15% and normal RVF. The patient was put on intravenous noradrenalin and two thoracic drains were inserted after consulting a cardiothoracic surgeon. Post-operatory chest x-ray demonstrated 2 well positioned thoracic drains. Blood and sputum cultures grew Pseudomonas aeruginosa, changing the antibiotic approach to piperacillin/tazobactam, improving his infectious status. Catecholamines in urine normalized and thyroid hormone levels were also normal.
After consulting a cardiothoracic surgeon, the patient was found to be unsuitable for ECMO. Advance Haemodynamic Monitoring with Pulse index Continuous Cardiac Output (PiCCO) revealed high cardiac output (hyper-dynamic heart) of 13L/min, low Systemic Vascular Resistance (SVR =500Ds/Cm5) and Systemic Vascular Resistance Index (SVRI =1276DS.m2/cm5), measures compatible with septic shock and Extravascular-Lung Water Index (ELWI=11.7 ml/kg), corresponding to pulmonary edema (Figure 1). Although lung Computed Tomography (CT) was the best course of action, the patient wasn't transportable due to need for NO maintenance and high doses of noradrenalin support.d and thyroid hormone levels were also normal.
Figure 1: Advance Haemodynamic Monitoring with Pulse index Continuous CarSdiac Output (PiCCO). Measures revealed high cardiac output (hyper-dynamic heart) of 13L/min, low SVR (500 Ds/cm5) & SVRI (1276DS.m2/cm5), all the measures were compatible with septic shock. ELWI (11.7ml/kg), compatible with wet lung (pulmonary edema).
SVR: Systemic Vascular Resistance; SVRI: Systemic Vascular Resistance Index; CO: Cardiac Output; CCI: Contour Cardiac Index; SV: Stroke Volume; GEDV: Global End Diastolic Volume; GEDI: Global End-Diastolic Index; ITBI: Intra-Thoracic Blood Index; ITBV: Intrathoracic Blood Volume in heart + pulmonary vessels; EVLW: Extravascular Lung Water – water content in lungs; ELWI: Extravascular Lung Water Index; SVV: Stroke Volume Variation; GEF: Global Ejection Fraction; PPV: Pressure Pulse Variation; Dpmax: slope of pressure vs time trace = contractility of LV.
On day 10, the patient stabilized and the left thoracic drain was removed. A new chest x ray performed revealed normal bilateral lung expansion. On day 11, the patient rapidly deteriorated with severe hypoxemia (PaO2 50 mmHg) and hemodynamic compromise. Additional left axillary midline thoracic drain was inserted with improvement in hemodynamic status and hypoxemia but lasted only minutes. Still in severe hypoxemia, a new left side anterior pneumothorax on chest x-ray has proven to be the source and a left anterior drain was inserted to a total of 3 thoracic drains.
A new chest x-ray showed right tension pneumothorax and another thoracic drain was inserted. CPR was performed for 25 minutes with intravenous administration of adrenalin, amiodarone and sodium-bicarbonate. Cardiac monitoring showed ventricular fibrillation, necessitating the administration of 2 more defibrillator shocks before return to sinus rhythm.
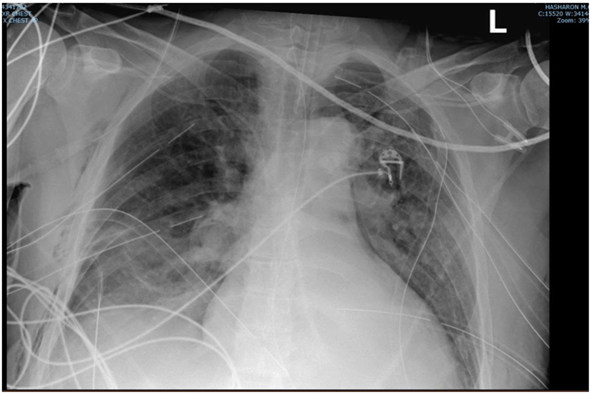
The day after, the patient's condition deteriorated rapidly again, and chest x-ray revealed a new right tension pneumothorax and additional thoracic drains were inserted, rapidly improving his condition. In total 7 thoracic drains were inserted (4 on the left side and 3 on the right side of the chest) (Figure 2).
Figure 2: Chest x-ray. Endotracheal tube 3.6cm above the carina. Multiple thoracic drain insertion- right side: 3 thoracic drains, left side: 4 thoracic drains.
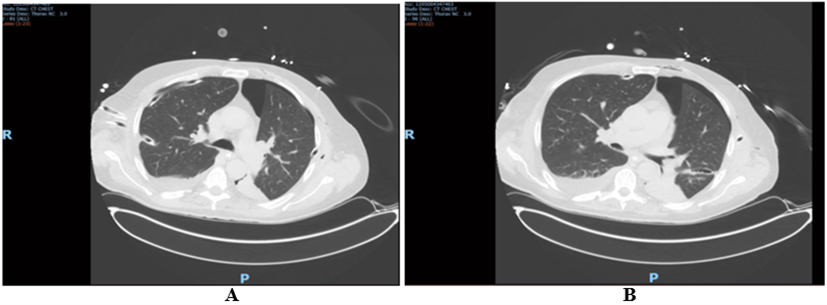
Figure 3: Axial computed tomography (CT) slides. A) Right side with 2 thoracic drains between 4-5 ribs; right inferior drain tip on the lung parenchyma; multiple consolidation right upper lobe. B) Left side with 2 thoracic drains; upper drain between 4-5 ribs; lower drain between 7-8 ribs, tip in the lung parenchyma; left upper pneumothorax not resolved after drain insertion.
On day 17, 5 thoracic drains were removed. The following chest x-ray showed full expansion of the lungs. P/F ratio has improved to 220 and NO was tapered down. On day 18, lung CT confirmed absence of pneumothorax in right side of the chest, with a residual small pneumothorax in left side (Figure 3). Brain CTA confirmed absence of hypoxemic brain injury.
On day 19, the patient underwent percutaneous tracheostomy. The 2 last thoracic drains were removed. He opened his eyes spontaneously after 23 days in ICU and was discharged to a rehabilitation center.
Discussion
We presented a case of a patient with severe cardiomyopathy induced by high doses methadone therapy who following CPR developed recurrent tension pneumothorax. Methadone, a synthetic opioid agonist of the mu receptor, is the mainstream treatment for opioids addiction. It is considered a safe drug but has many side effects in high doses. Skin, gastrointestinal, central nervous system and cardiovascular systems may all be affected.
QT interval prolongation and dispersion in our patient could have led to wide complex tachycardia and most probably VT. Abramson et al. showed that daily use of methadone could increase the QT interval by 12 msec [3]. Krantz et al. showed that in addition to QT interval prolongation other cardiac repolarization abnormalities can also manifest as QT interval dispersion [4].
Our patient received high doses (120 mg) of methadone daily for heroin use. Because of wide complex tachycardia and diffuse ST elevation on ECG, he was admitted to ICCU. TTE reveled LVEF around 20% (severe LV dysfunction) but apical akinesia was never mentioned, and coronarography demonstrated patent coronaries, ruling out stress methadone-withdrawal induced cardiomyopathy.
Walker et al. as well described three patients that presented with TdP after prolonged treatment of high dose of methadone (more than 300 mg daily) [5]. However, this is usually seen in QT interval longer than 500 msec, not present in our case. There were no electrolytes abnormalities including magnesium.
In fact, his cardiac output was very high correlating with sepsis, and catecholamines release is known after opioids withdrawal [6]. Following treatment with broad spectrum antibiotics, addition of noradrenaline, vasopressin and steroids allowed his cardiac output to normalize. Critically ill patients in the ICU are at high risk of developing pneumothoraces as a complication of barotrauma from mechanical ventilation or due to insertion of central vascular lines. Patients with ARDS have a higher prevalence of pneumothorax [7].
Our first intention to treat was guided by a consultation with a cardiothoracic surgeon regarding severe LV dysfunction and pulmonary edema. Possibility of ECMO venoarterial or veno-venous (VA; V-V) was also considered. Rapid deterioration of his condition, CPR and recurrent bilateral tension pneumothorax (5 more thoracic drain insertions) obliged us to reconsider our therapeutic approach.
The use of VA-ECMO was described in the literature to resolve opioids induced pulmonary edema [8]. In our case, after consulting a cardiothoracic surgeon we could not perform VA-ECMO as bridge therapy because the patient was not allegeable for heart-lung transplant.
Due to positive intrathoracic pressure in a mechanically ventilated patient, all pneumothoraces should be drained to avoid tension pneumothoraces. Also, recurrent pneumothoraces occur commonly in mechanically ventilated patients with ARDS despite thoracic drain insertion [9]. Notice that in our case, while performing CPR and because of the rapidly degrading situation of our patient, since the diagnosis of tension pneumothoraces is made clinically, we could not perform chest x-ray instead of continuing resuscitation, so only after stabilization of his condition we performed chest x-ray showing new tension pneumothoraces and a few days later lung CT.
Concerning the patient's hemodynamic status, PiCCO revealed measures corresponding to septic shock, especially concerning the low SVR/SVRI but also high cardiac output and stroke volume. Interesting was also the value of the contractility of his heart and low Global ejection fraction (GEF) but also the value of the GEDI which corresponds to his very low LVEF (~15%) and severe cardiomyopathy.
Also, EVLWI value was high corresponding to hydrostatic (cardiogenic) pulmonary edema (pulmonary vascular permeability index PVPI 1.6). Interpreting PiCCO values after cardiac arrest can be challenging and still has not been validated. Tagami in his study of 90 patients admitted to the ICU after cardiac resuscitation showed that trans-pulmonary thermodilution monitoring was precise and accurate even in therapeutic hypothermic patients [10].
After cardiac arrest and CPR, there are many changes in hemodynamics and respiratory status, haemodynamics monitoring is of great interest and PiCCO measures can be used in monitoring the condition of those patients, which also contributed to guide and improve the status of our patient [11].
Conclusion
We described a case of a 56-year-old patient suffering from cardiomyopathy and severe LV dysfunction consecutive to high-dose methadone treatment given for heroin abuse who developed septic and cardiogenic shock refractory to treatment. Our experience suggests that a multimodal therapy including CPR, multiple thoracic drains insertion, high doses of vasopressors and haemodynamic monitoring to guide therapy are necessary. On twenty-ninth day of hospitalization, the patient was discharged to a step down ward with no further requirement for ventilator or haemodynamic support.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Author Consent
All the authors agree with the publication.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Case ReportPublication history
Received: Wed 28, Sep 2022Accepted: Thu 20, Oct 2022
Published: Thu 12, Jan 2023
Copyright
© 2023 Yaniv Hadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JCMCR.2022.01.02
Author Info
Olivier Zerbib Yaniv Hadi Daniel Kovarsky Gal Sahaf Levin Tamar Gottesman Mor Darkhovsky Shaul Lev
Corresponding Author
Yaniv HadiAzrieli Faculty of Medicine, Bar-Ilan University, Israel
Figures & Tables

SVR: Systemic Vascular Resistance; SVRI: Systemic Vascular Resistance Index; CO: Cardiac Output; CCI: Contour Cardiac Index; SV: Stroke Volume; GEDV: Global End Diastolic Volume; GEDI: Global End-Diastolic Index; ITBI: Intra-Thoracic Blood Index; ITBV: Intrathoracic Blood Volume in heart + pulmonary vessels; EVLW: Extravascular Lung Water – water content in lungs; ELWI: Extravascular Lung Water Index; SVV: Stroke Volume Variation; GEF: Global Ejection Fraction; PPV: Pressure Pulse Variation; Dpmax: slope of pressure vs time trace = contractility of LV.
References
1. Saiful FB, Lafferty
J, Jun CH, Teli S, Duvvuri S et al. (2011) Takotsubo cardiomyopathy due to
iatrogenic methadone withdrawal. Rev Cardiovasc Med 12: 164-167. [Crossref]
2. Lemesle F, Lemesle
F, Nicola W, Bera APJ (2010) First case of stress cardiomyopathy as a result of
methadone withdrawal secondary to drug-drug interaction. Am J Emerg Med
28: 387.e5-387.e6. [Crossref]
3. Abramson DW, Quinn
DK, Stern TA (2008) Methadone-Associated QTc Prolongation: A Case Report and
Review of the Literature. Prim Care Companion J Clin Psychiatry 10:
470-476. [Crossref]
4. Krantz MJ, Lowery
CM, Martell BA, Gourevitch MN, Arnsten JH (2005) Effects of methadone on QT‐interval
dispersion. Pharmacotherapy 25: 1523-1529. [Crossref]
5. Walker PW, Klein D,
Kasza L (2003) High dose methadone and ventricular arrhythmias: a report of
three cases. Pain 103: 321-324. [Crossref]
6. Garofalo NA, Neto
FJT, Pereira CDN, Pignaton W, Vicente F et al. (2012) Cardiorespiratory and
neuroendocrine changes induced by methadone in conscious and in isoflurane
anaesthetised dogs. Vet J 194: 398-404. [Crossref]
7. Moss HA, Roe PG,
Flower CD (2000) Clinical deterioration in ARDS - an unchanged chest radiograph
and functioning chest drains do not exclude an acute tension pneumothorax. Clin
Radiol 55: 637-639. [Crossref]
8. Daugherty LE (2011)
Extracorporeal membrane oxygenation as rescue therapy for methadone-induced
pulmonary edema. Pediatr Emerg Care 27: 633-634. [Crossref]
9. Heffner JE,
McDonald J, Barbieri C (1995) Recurrent pneumothoraces in ventilated patients
despite ipsilateral chest tubes. Chest 108: 1053-1058. [Crossref]
10. Tagami T, Kushimoto S, Tosa R, Omura M, Hagiwara J et al. (2012) The precision of PiCCO® measurements in hypothermic post-cardiac arrest patients. Anaesthesia 67: 236-243. [Crossref]
11. Monnet X, Anguel N, Osman D, Hamzaoui O, Richard C et al. (2007) Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med 33: 448-453. [Crossref]
