Multilocular Cystic Nephroma: Is Radical Nephrectomy The Best Treatment In All Cases? Our Experience After 45 Years Working on a Tertiary Care Center
A B S T R A C T
Introduction: Multilocular cystic nephroma (MCN) is a type of benign renal tumor that is sometimes misdiagnosed with cystic Wilms Tumor. Given that each one has a different treatment, it is essential to make a proper diagnosis.
Objective: Our aim was to find the differential characteristics of MCN and to be able to identify these renal masses with low probability of malignancy, which are subsidiaries of a more conservative treatment.
Material and Methods: We conducted a retrospective descriptive study were we analyzed the patients with MCN treated in our center between 1971 and 2016.
Results: We found 13 patients with a histological diagnosis of MCN, mostly males, with an average age of 46.8 months at the time of surgery. Clinically, 2 cases (15.4%) were asymptomatic, 6 had an abdominal mass (46.1%), 3 had abdominal pain (23.1%) and 2 had hematuria (15.4%). The maximum diameter of the mass was 7.3 cm (mean). 9 nephrectomies (69.2%) and 4 tumorectomies (30.8%) were performed. In two of these (15.4%), neoadjuvant chemotherapy was administered due to presurgical suspicion of Wilms' tumor, which was discontinued after the pathological analysis. In another 4 patients with suspicion of MCN, all of them asymptomatic and with a tumor size <3cm, non-surgical treatment was decided. At the present time all patients (operated or not) stay alive. No. cases of distant metastasis, local recurrence or mass progression (in the non-operated patients) have been recorded.
Conclusion: Given its good prognosis, MCN can be treated with a non-radical surgery or even non-surgical conservative treatment in selected cases. In our experience, the clinical and radiological characteristics are the pillars to identify those patients with low risk of malignancy in which this attitude could be carried out.
Keywords
Complex renal mass, multilocular cystic nephroma, nephrectomy, nephroblastoma, renal cyst
Introduction
Renal tumors are rare in children. Nephroblastoma - or Wilms Tumor (WT)–is the most frequent within this group [1, 2]. Multilocular Cystic Nephroma (MCN) is a type of benign renal tumor that in most cases does not produce symptoms and has a good prognosis [3, 4]. However, it is sometimes misdiagnosed with cystic WT and treated as if it was malignant [5]. Given that the treatment of TW is chemotherapy and surgery and that the treatment of MCN is less aggressive, a correct pretreatment diagnosis of the mass is essential.
If we are able to predict the histological type, based on the clinical manifestations and the radiological aspect, the presurgical diagnosis could influence the therapeutic strategy. The objective of this work is, therefore, to analyze the clinical and radiological characteristics of the MCN in order to establish the criteria that allow us to identify those masses with low probability of malignancy, which could receive a conservative treatment.
Material and Methods
Retrospective descriptive study of all patients diagnosed with MCN (confirmed by pathology) in our centre, between 1971 and 2016. Initially, there was a sample of 17 cases with diagnostic suspicion of MCN, of which 13 finally made up our group of study. These 4 cases were excluded due to the decision of non-surgical treatment and the absence of histological confirmation. In order to characterize the cases of MCN treated in our center during this period, clinical histories were reviewed, analyzing the demographic data, presence of symptoms, diagnostic tests used, type of treatment chosen and patients’ follow-up.
Results
Thirteen patients with a pathology diagnosis of MCN were found (Table 1). Nine of them (69.2%) were male. The mean age at the time of surgery was 46.8 months (range 1-153); but excluding the 3 cases that exceeded the age of 10 years, the median age was 19.6 months (range 1-54). Clinically, 2 cases (15.4%) were asymptomatic at the time of diagnosis. The tumor was incidentally diagnosed in both during the routine physical examination at the first outpatient visit for hypospadias. The rest of the patients presented with symptoms: 6 cases had abdominal mass (46.1%), 3 had abdominal pain (23.1%) and 2 had hematuria (15.4%). None of the tumors was suspected prenatally, not even in the case that was operated at the age of one month.
Table 1: Characteristics of our study group.
|
# |
Sex |
Age (months) |
Symptoms |
Maximum size ( cm) |
Surgery |
CT |
|
1 |
M |
143 |
Pain |
5 |
Tumorectomy |
No |
|
2 |
F |
22 |
Hematuria |
9 |
Nefrectomy |
Yes |
|
3 |
M |
21 |
Mass |
9 |
Nefrectomy |
No |
|
4 |
F |
54 |
Pain |
5,9 |
Tumorectomy |
No |
|
5 |
M |
21 |
Mass |
8,5 |
Nefrectomy |
No |
|
6 |
F |
153 |
Pain |
3,2 |
Tumorectomy |
No |
|
7 |
M |
1 |
Mass |
10 |
Nefrectomy |
No |
|
8 |
F |
13 |
Mass |
7,5 |
Nefrectomy |
No |
|
9 |
M |
117 |
- |
8 |
Nefrectomy |
No |
|
10 |
M |
14 |
Mass |
unknown |
Nefrectomy |
No |
|
11 |
M |
15 |
Hematuria |
unknown |
Nefrectomy |
No |
|
12 |
M |
20 |
Mass |
unknown |
Nefrectomy |
Yes |
|
13 |
M |
15 |
- |
unknown |
Tumorectomy |
No |
M: male, F: female, CT: chemotherapy.
Figure 1: Sonographic images. A) Great cystic renal mass with multiple thin septa, with no solid elements, occupying practically the entire kidney. B) Localized cystic kidney mass.
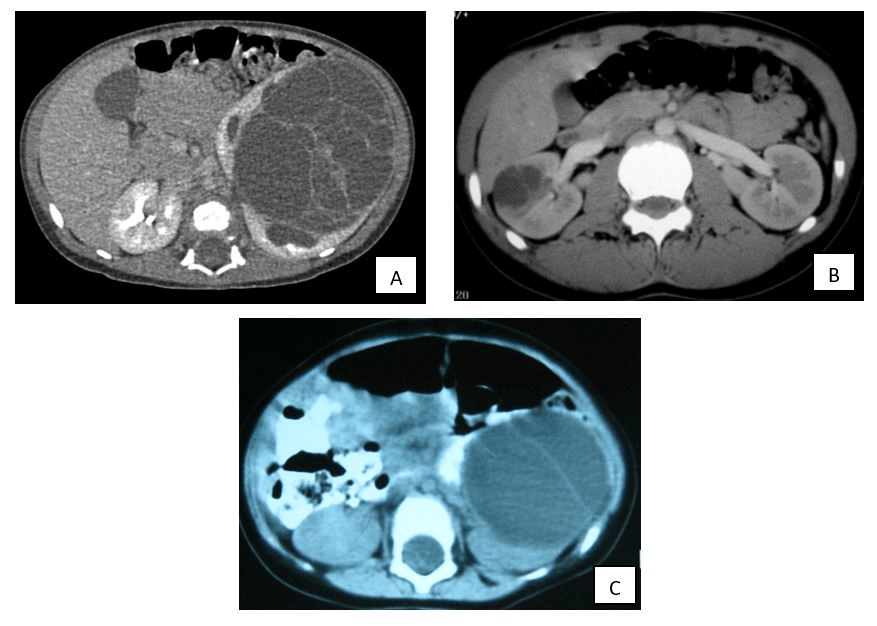
In all patients, the initial imaging test was abdominal ultrasound (Figure 1), completed with computerized tomography (CT, Figure 2) in 12 cases (92.3%) and magnetic resonance imaging (MRI) in the latter. Radiologically, renal masses had multiple cysts separated by septa with absence of solid elements inside. In addition, two of them (15.4%) associated small calcifications. The maximum diameter of the renal mass ranged between 3.2 and 10 cm (mean 7.3 cm), with an average volume of 230 cc (range 2.5-540). Radical surgery was performed in all the cases that presented with an abdominal mass or hematuria, when WT was suspected. Those in whom, either because of their size or because of the clinical onset (abdominal pain or asymptomatic patient), the suspicion of malignancy was low, a more conservative surgery was performed.
Figure 2: CT images. A and B correspond to the images obtained by tomography of lesions A and B seen by ultrasound at Figure 1. C) Large renal lesion due to, essentially, two big cysts. Note: comparing A and C, the variable number of cysts that can take part of the MCN.
In total, 9 nephrectomies (69.2%) and 4 tumorectomies (30.8%) were performed. One nephrectomy also associated adrenalectomy and lymph node biopsy due to suspicion of WT. In two cases (15.4%), neoadjuvant chemotherapy (protocol for WT) was administered, but after the surgery it was discarded by the pathologist. None of the patients in our sample received adjuvant chemotherapy once the diagnosis of MCN was confirmed. With regard to the 4 patients in whom non-surgical treatment was decided, periodic clinical and radiological monitoring is being carried out (every 6-12 months). Two of them are twin sisters and the size of the renal mass did not exceed 3 cm in any of these 4 cases. In all the patients, the parents preferred not to operate given the suspicion of a benign tumor and the absence of symptoms.
At the present time, all the operated patients are alive and free of disease and there has not been any case of distant metastasis or local recurrence. On the other hand, the 4 patients in whom surgery was not elected, after a 3 to 5 years of follow-up, are clinically and radiologically stable.
Discussion
Renal tumors are rare in the pediatric age, highlighting nephroblastoma-or Wilms tumor (WT)-, which constitutes 6-7% of tumors in children and the 90% of renal masses within this age group [1, 2, 6]. It is followed in frequency by clear cell renal sarcoma, rhabdoid tumor, clear cell carcinoma, congenital mesoblastic nephroma and multilocular cystic nephroma (MCN) [2, 7]. The MCN is a benign renal tumor of congenital nature, which represents approximately 5% of childhood kidney tumors. 80% of patients are 3 to 24 months, with a higher prevalence in men (65%), as it was obtained in our sample [3]. On the contrary, WT has an average age of presentation of 3.5 years and affects both sexes equally. They are usually unilateral, although familiar and bilateral cases have been described [2].
This tumor (MCN) is usually diagnosed by chance, when performing imaging studies for other causes, since patients are asymptomatic or present anodyne or nonspecific symptoms in most cases [3, 4]. This data contrasts with what was found out in our study, where only 2 cases did not present symptoms, which could translate to the fact that the incidence of MCN in asymptomatic population could be underestimated. However, and as in the vast majority of our patients, some may be diagnosed due to abdominal mass or hematuria and may be confused with other malignant strains, causing a more aggressive and closer management [3, 8]. It is believed that the origin of this tumor line is a consequence of the premature cessation of the branching of the ureteral bud, so that the metanephros-inducing activity would be reduced or paralyzed -as occurs in dysplasia [8].
The first reference in the literature of an MCN was made by Edmunds in 1892 and, in 1951, Powell et al. established the necessary criteria for its diagnosis [8, 9]. These were: unilateral affectation, single lesion, multilocular lesion, absence of communication between cavities, absence of communication of the cysts with the renal pelvis, epithelial-lined cysts, absence of nephrons in the separation walls of the cysts and normality of the remaining parenchyma. These criteria have been modified by Joshi and Beckwith in more recent articles where they emphasize that in an MCN the only solid tissue found are the thin partitions that separate the cysts, being able to lodge renal tubules well developed, but never complete nephrons [5]. The cysts usually contain a clear liquid, with a chemical composition similar to blood serum and normal cytological characteristics. The diameter of these cysts can vary from a few millimeters to several centimeters (Figure 3).
Figure 3: Microscopic images of MCN (H & E). It characteristically presents multiple cysts of thin and regular walls, covered by cuboidal cells of eosinophilic cytoplasm and pyknotic nuclei, with walls of fibro-connective elements. Absence of atypia, necrosis or calcification. The fundamental characteristic that differentiates WT from MCN is the presence of solid expansive regions of nephroblastomatous tissue not modeled by cystic spaces.
Due to its limited knowledge, its low incidence and its similarity with the WT, the MCN is often confused with it. In fact, renal cystic lesions include MCN, partially differentiated nephroblastoma and cystic Wilms tumor (CWT) [2, 5, 10]. All three can be part of a spectrum where the MCN is a benign lesion, the CWT is a malignant lesion and in the intermediate zone there is partially differentiated nephroblastoma (PDN) [10]. Hence the importance of a correct diagnosis. Although the diagnosis of certainty is pathology analysis, radiology is a fundamental pillar in the diagnostic process, with ultrasound and CT and / or MRI being the main tools used in the main pediatric centers, such as ours. Fine needle aspiration (FNA) of the cysts is not indicated because they are independent of each other, which would require individual puncture of all of them besides the risk of bleeding, infection or tumor spread and the fact that the negativity for malignant cells would not exclude the tumoral origin [11].
According to the current oncological protocols of the International Society of Pediatric Oncology (SIOP; UMBRELLA SIOP–RTSG 2016 protocol), WT is treated preoperatively with chemotherapy, while the MCN is managed only with surgery [7]. The key point here is to predict the histological type based on the clinical manifestations and its radiological aspect, since in this case the pre-surgical diagnosis would influence the therapeutic strategy. In connection with this, Bosniak proposed in 1986 a classification of renal cystic lesions, which was posteriorly evaluated and modified by himself [12-14].
I. Class I: Simple benign cyst.
II. Class II: Minimally complicated cystic lesions, with presence of fine septa and/or small calcifications, clusters of cysts or hyperdense (+20UH) or infected cysts.
i. Class IIF (F="follow"): Cystic lesion, minimally complicated but with some suspicious sign of malignancy that makes it closer to group III (greater thickness of wall or gross calcifications).
III. Class III: Complex cystic lesions (greater calcifications, irregular margin, thick septa). In approximately 50% of cases, we will find a malignancy.
IV. Class IV: Cystic carcinoma. Lesions with radiological findings of malignancy (cystic or necrotic components and solid elements).
Type I cystic lesions, fortunately, are the most frequent subtype and do not offer diagnostic doubts. The same applies to subtype IV. However, subtypes II and III are the ones that cause the major problems, hence the subtype IIF was subsequently added [12-14]. Classically, the MCN has been classified into one of these two groups, usually type III, so radical surgery has been the treatment of choice in these cases given the high probability of malignant mass with these characteristics [3]. In contrast to this, some cases treated with conservative surgery have been described (bilateral involvement, single patient, small lesions) or even expectant treatment -with clinical and radiological follow-up- with a good evolution [4, 8, 10].
This more conservative attitude has been chosen in our center in selected cases, as asymptomatic patients or with nonspecific symptoms -abdominal pain- and small masses, where we performed tumorectomy. Also, in the 4 cases that were offered close clinical-radiological follow-up and, therefore, were not included in our sample due to lack of histological sample for diagnostic confirmation. In this last group, the criteria that we have followed to not operate are: absence of clinical symptoms, tumor size less than 3cm and stability during follow-up, consensus with the parents of the non-surgical decision and regular clinical and ultrasound control (each 6-12 months). The prognosis of MCN is good, with low or no local recurrence or metastasis rate [3, 4]. This coincides with that observed in our study throughout the follow-up, with no cases of recurrence or metastasis.
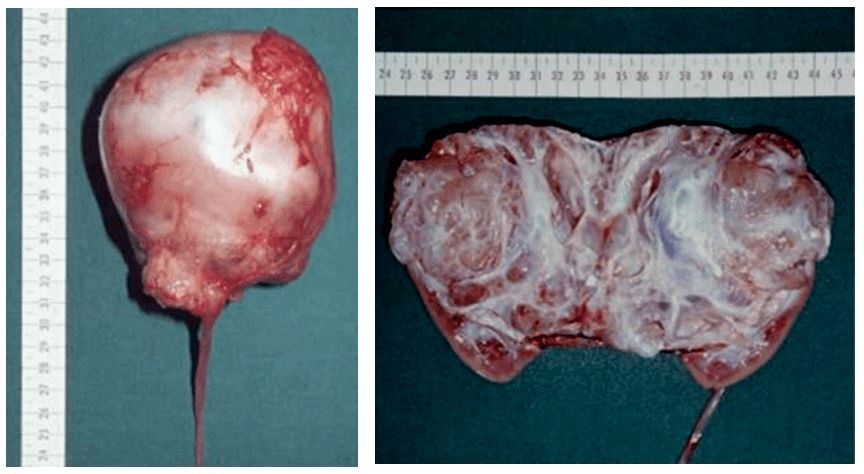
Summary Figure: Macroscopic image of MCN after radical nephrectomy.
For all these reasons, and as has already been proposed in other publications, we consider that the classification of Bosniak, although it is very useful, should not be an absolute criteria for surgery but rather be a guiding tool [4]. The size of the tumor mass, the evolution and clinical manifestations as well as the renal function of the patient are data that should be taken into account when deciding the ideal treatment in each specific case (radical nephrectomy, partial nephrectomy, tumorectomy, expectant treatment).
Therefore, we propose modifying the current protocol for the treatment of MCN, performing conservative surgery instead of radical nephrectomy in as much cases as possible and even choosing a non-surgical management in those patients who are asymptomatic, stable and with small tumor size. However, we must remember that the histological study is the only effective method to differentiate the MCN from malignant renal cystic lesions, mainly the TW, so that close clinical-radiological long term follow-up is essential in those patients in whom expectant treatment is the option [3].
As limitations, it should be noted that this is a retrospective study, consisting of a series of cases. Although the follow-up period is extensive, there have been changes throughout the years in terms of medical staff, treatment protocols and technological quality. Therefore, it would be convenient to carry out prospective, multicenter studies that provide more reliable information in this regard.
Conclusion
Although due to its low prevalence and its characteristics MCN is sometimes confused with WT, it is a benign renal tumor with a good prognosis following non-radical surgery or even conservative (non-surgical) treatment in selected cases. In our experience, clinical and radiological characteristics are the fundamental pillars to identify those patients with low risk of malignancy in which this attitude could be carried out.
Conflicts of Interest
None.
Funding
None.
Article Info
Article Type
Research ArticlePublication history
Received: Sat 28, Mar 2020Accepted: Sat 11, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 P. Ortolá Fortes. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COCB.2020.01.03
Author Info
Agustín Serrano Durbá Anna Sánchez Llopis Cinta Sangüesa Nebot Domínguez Hinarejos Carlos M. A. Conca Baenas March Villalba José Antonio P. Ortolá Fortes Polo Rodrigo Alba
Corresponding Author
P. Ortolá FortesPediatric Urology Service, Hospital Universitario and Politécnico La Fe, Valencia Spain
Figures & Tables
Table 1: Characteristics of our study group.
|
# |
Sex |
Age (months) |
Symptoms |
Maximum size ( cm) |
Surgery |
CT |
|
1 |
M |
143 |
Pain |
5 |
Tumorectomy |
No |
|
2 |
F |
22 |
Hematuria |
9 |
Nefrectomy |
Yes |
|
3 |
M |
21 |
Mass |
9 |
Nefrectomy |
No |
|
4 |
F |
54 |
Pain |
5,9 |
Tumorectomy |
No |
|
5 |
M |
21 |
Mass |
8,5 |
Nefrectomy |
No |
|
6 |
F |
153 |
Pain |
3,2 |
Tumorectomy |
No |
|
7 |
M |
1 |
Mass |
10 |
Nefrectomy |
No |
|
8 |
F |
13 |
Mass |
7,5 |
Nefrectomy |
No |
|
9 |
M |
117 |
- |
8 |
Nefrectomy |
No |
|
10 |
M |
14 |
Mass |
unknown |
Nefrectomy |
No |
|
11 |
M |
15 |
Hematuria |
unknown |
Nefrectomy |
No |
|
12 |
M |
20 |
Mass |
unknown |
Nefrectomy |
Yes |
|
13 |
M |
15 |
- |
unknown |
Tumorectomy |
No |
M: male, F: female, CT: chemotherapy.


References
- Steliarova-Foucher E, Stiller C, Kaatsch P, Berrino F, Coebergh JW et al. (2004) Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet 364: 2097-2105. [Crossref]
- Ehrlich Pf, Shamberger RC (2014) Renal tumors. In: Ashcraft's Pediatric Surgery. Ed: Holcomb III GW, Murphy JP, Ostlie DJ. 6ª Ed. Elsevier 859-882.
- Chang CP, Li JR, Yang CS, Ou YC, Cheng CL (2014) Multilocular cystic nephroma: A case report and review of the literature. Urological Science 25: 109-111.
- Granja MF, O’Brien AT, Trujillo S, Mancera J, AguirreD (2015) Multilocular Cystic Nephroma: A Systematic Literature Review of the Radiologic and Clinical Findings. AJR Am J Roentgenol 205: 1188-1193. [Crossref]
- Joshi VV, Beckwith JB (1989) Multilocular cyst of the kidney (cystic nephroma) and cystic, partially differentiated nephroblastoma. Terminology and criteria for diagnosis. Cancer 64: 466-479. [Crossref]
- Pastore G, Znaor A, Spreafico F, Graf N, Pritchard-Jones K et al. (2006) Malignant renal tumours incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 42: 2103-2114. [Crossref]
- van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R et al. (2017) Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP–RTSG 2016 protocol. Nat Rev Urol 14: 743-752. [Crossref]
- Powell T, Shackman R, Johnson HD (1951) Multilocular cysts of the kidney. Br J Urol 23: 142-152. [Crossref]
- Edmunds W (1892) Cystic adenoma of the kidney. Trans Pathol Soc Lond 43: 89-90.
- Cozzi F, Morini F, Schiavetti A, Catalano C, Bosco S et al. (2003) Enucleative Surgery in an Infant With Giant Cystic Nephroma. J Urol 169: 1493-1494. [Crossref]
- Bosniak MA (1993) Problems in the radiologic diagnosis of renal parenchymal tumors. Urol Clin North Am 20: 217-230. [Crossref]
- Bosniak MA (1986) The current radiological approach to renal cysts. Radiology 158: 1-10. [Crossref]
- Bosniak MA (1997) Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR Am J Roentgenol 169: 819-821. [Crossref]
- Bosniak MA (1997) The use of the Bosniak classification system for renal cysts and cystic tumors. J Urol 157: 1852-1853. [Crossref]
