Multicystic Alveolar Adenoma in a Symptomatic Adult with Extensive Bullae and Mediastinal Shift
Multicystic Alveolar Adenoma in a Symptomatic Adult with Extensive Bullae and Mediastinal Shift
A B S T R A C T
We present a unique case of alveolar adenoma with extensive bullous disease in a young female patient that presented as left sided pleuritic chest pain and shortness of breath. The patient had a pneumothorax and mediastinal shift. She underwent upper lobectomy that resulted in relative a resolution of the mediastinal shift. We discuss the unique clinical and pathologic presentation of this rare benign neoplasm in this patient.
Keywords
Alveolar adenoma, multicystic lung disease, mediastinal shift
Introduction
Alveolar adenoma was first reported in the literature in 1986 as a benign neoplasm comprised of both alveolar epithelium and mesenchyme [1]. Since then, alveolar adenomas are still rarely reported in the literature with approximately only 40 cases reported [2]. The most common presentation is of an asymptomatic solitary, peripheral lesion that is incidentally discovered on chest imaging [2-6]. Alveolar adenomas rarely present as a solitary cystic lesion [4, 6-8]. Cystic and non-cystic alveolar adenomas are found incidentally without symptoms. The largest cystic alveolar adenoma was a solitary 91x50x98-mm multiseptated lingula mass in a 38-year-old female presenting with dyspnea [8]. There has yet to be a case in the literature where alveolar adenoma was associated with an extensive multifocal bullous disease throughout an entire lobe leading to a shift of the mediastinum.
Case Description
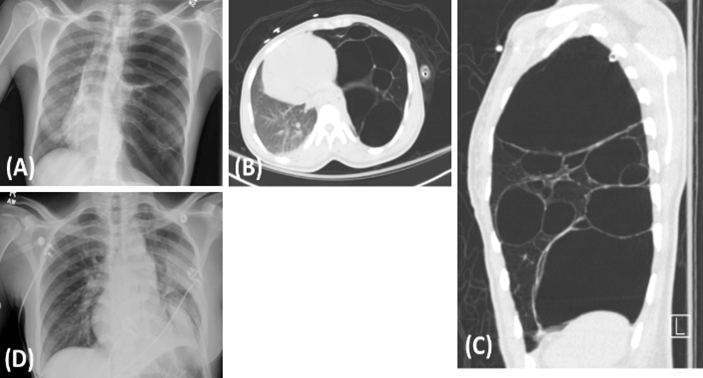
A 26-year-old woman with a history of a left-sided pneumothorax presented with a sensation of breathlessness, associated with lightheadedness and distal paresthesia. She admitted to having similar episodes in the past and underwent placement of a chest tube 6 years prior to presentation at an outside hospital. She denied any other relevant medical or family history. Her exam was significant for decreased breath sounds on the left side. Initial chest X-ray showed a left sided pneumothorax, blebs, and a mediastinal shift to the right (Figure 1A). She had a left chest tube placed, and a computed tomography scan of the chest showed extensive bullous disease of the left upper lobe (Figures 1B & 1C). The differential diagnosis included congenital pulmonary airway malformation (CPAM), lymphangiomyomatosis, and congenital lobar emphysema. The patient underwent a left upper lobectomy, and the resulting specimen was sent for pathological examination (Figure 2). A follow-up chest X-ray showed a relative resolution of the mediastinal shift (Figure 1D). She recovered without incident and was discharged on hospital day 6.
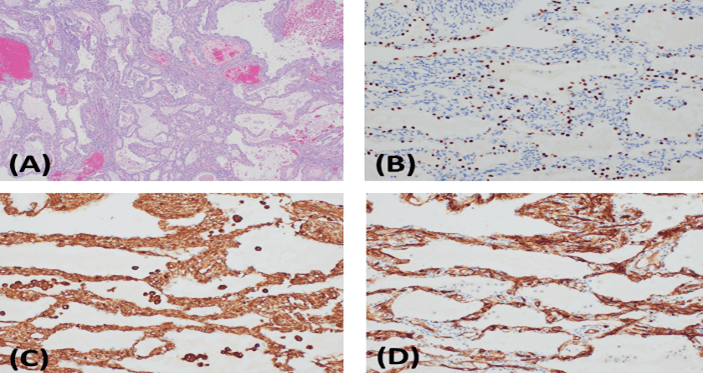
Macroscopically, the lung was hyperinflated with multiple thin-walled cystic bullous lesions. The majority of the cysts were air-filled, and a few contained gelatinous tan-red material. The lining of the cysts was predominantly smooth with focal areas of blue-gray excrescences measuring up to 3.2 cm maximum in diameter. Microscopically, the areas of blue-gray excrescences corresponded to a well-demarcated area composed of small cystic spaces filled with proteinaceous material and lined by small cuboidal epithelial cells. The septae between cyst walls were variable, with bland stromal cells and sparse inflammatory cell infiltrate and occasional lymphoid aggregates. The bullae and cysts were unencapsulated but well demarcated from the adjacent, normal-appearing lung (Figure 3A). The epithelial cells lining the cystic spaces were strongly positive for CK7, EMA and TTF-1, while negative for vimentin, CD34, and all other mesenchymal markers (Figures 3B-3D). On the contrary, the stromal cells were positive for vimentin, CD34, and SMA (focal), while negative for all other epithelial markers. Both cell populations were negative for CD31, S100, HMB-45, ER, PR, desmin, calponin, and p63. The morphological appearance and immunohistochemical stains were most consistent with an alveolar adenoma.
Figure 1: Radiographic Imaging. A) CXR upon presentation with visible cystic regions in the left lung and mediastinal shift with compression of the right lung. B) & C) Post-chest-tube CT demonstrates the large cystic nature of the malformation and the compression of the mediastinum and right lung. D) CXR prior to discharge shows relative resolution of mediastinal shift and re-expansion of the right lung.
Figure 2: Intraoperative Findings. A) Intraoperative image shows the gross cystic deformity with adjacent relatively healthy appearing tissue. B) The specimen was approximately 11 cm in diameter.
Figure 3: Pathologic Examination Results. A) Low power H&E stained section shows the lesion is composed of primarily small cystic spaces filled with proteinaceous debris. Fresh blood is presumed to be procedural artifact as there was no evidence of chronic bleeding (4x). Immunohistochemical stains demonstrate two cell populations with epithelial markers and TTF-1 staining B) the cuboidal lining cells while C) vimentin and D) CD34 stain the stromal cells (20x).
Comment
We present a case of alveolar adenoma with multiple features not previously reported in the literature. This neoplastic lesion is almost always an asymptomatic solitary, peripheral lesion [2-5]. This case is unusual in both the clinical and pathological presentations. Our patient was severely symptomatic with multifocal bullous disease involving the majority of the left lobe, significant enough to cause a contralateral shift of the mediastinum and collapse of the uninvolved ipsilateral lobe. It had histopathological features similar to prior cases reported in the literature with both mesenchymal and epithelial components that define this diagnosis [1, 2, 4, 6]. While it is an exceptional presentation, the diagnosis of alveolar adenoma was confirmed on pathological examination.
There is one other case in the literature that presented with dyspnea and a large cystic mass, but it was solitary and not nearly as extensive as this case [8]. The pathophysiology of alveolar adenomas becoming cystic is not known. It has been hypothesized that it is caused by a unidirectional check-valve mechanism resulting in air trapping [8]. This check-valve hypothesis cannot completely explain the multifocal bullous disease found in our patient. The presentation of our patient suggests the destruction of the normal lung parenchyma leading to bullous formation.
Given the rarity of alveolar adenoma, there has been limited opportunities to characterize the presentation and pathology of this neoplasm [2]. It is likely underdiagnosed and the differential for these lesions can be broad. If presenting as a solid lesion, potential pathologies include papillary adenoma, sclerosing hemangioma, lymphangioma, or hamartoma. If presenting as a cystic lesion, the differential includes congenital cystic adenomatoid malformation, lymphangiomyomatosis, endometriosis, and bullous disease with granulation tissue [2]. In our case, there was no evidence of a bronchial respiratory epithelial lining or of smooth muscle bundles associated with the cyst walls to suggest CPAM. Alveolar variants of CPAM are described, but not with large bullous cysts. The cystic areas did not morphologically resemble endometrial tissue, and the positive TTF-1 and negative ER immunohistochemistry stains further argue against the possibility of endometriosis. Likewise, the negative HMB-45, ER and PR immunohistochemistry results argue against the possibility of lymphangiomyomatosis. Given the CT findings, there was initial consideration for a diagnosis of CPAM, but this disease is rarely seen in adults and has pathological features of bronchiolar and papillary epithelial proliferation, which was not found [2, 6]. This highlights the importance of careful and complete pathological examination and the relative ease at which the rarity of alveolar adenoma may lead to its underdiagnosis.
In this case, the pathology findings consistent with alveolar adenoma did not alter this patient’s management. Given the severity of her symptoms and the level of lung compression, she would have required surgical intervention regardless if the diagnosis was known preoperatively, although had this alveolar adenoma presented more typically, as a small isolated lung lesion, it could have been managed with a simple wedge resection.
This is a case of alveolar adenoma with clinical and pathological features not previously reported. This adds to the literature by expanding the known features of this seldom reported benign neoplasm. It also highlights the difficulty in diagnosing this rare lesion through imaging and stresses the importance of surgical excision and postoperative histopathology and immunohistochemistry for proper diagnosis.
Funding
None.
Conflicts of Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Sat 29, Feb 2020Accepted: Fri 20, Mar 2020
Published: Fri 16, Oct 2020
Copyright
© 2023 Lindsay Volk. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.03.03
Author Info
Lindsay Volk Christine Minerowicz Siavash Saadat John E. Langenfeld
Corresponding Author
Lindsay VolkDivision of Cardiothoracic Surgery, Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA
Figures & Tables

References
- Yousem SA, Hochholzer L (1986) Alveolar adenoma. Hum Pathol 17: 1066-1071. [Crossref]
- Wang X, Li WQ, Yan HZ, Li YM, He J et al. (2013) Alveolar adenoma combined with multifocal cysts: case report and literature review. J Int Med Res 41: 895-906. [Crossref]
- Fujimoto K, Muller NL, Sadohara J, Harada H, Hayashi A et al. (2002) Alveolar adenoma of the lung: computed tomography and magnetic resonance imaging findings. J Thorac Imaging 17: 163-166. [Crossref]
- Cavazza A, Paci M, De Marco L, Leporati G, Sartori G et al. (2004) Alveolar adenoma of the lung: a clinicopathologic, immunohistochemical, and molecular study of an unusual case. Int J Surg Pathol 12: 155-159. [Crossref]
- Sak SD, Koseoglu RD, Demirag F, Akbulut H, Gungor A (2007) Alveolar adenoma of the lung. Immunohistochemical and flow cytometric characteristics of two new cases and a review of the literature. APMIS 115: 1443-1449. [Crossref]
- Burke LM, Rush WI, Khoor A, Mackay B, Oliveira P et al. (1999) Alveolar adenoma: a histochemical, immunohistochemical, and ultrastructural analysis of 17 cases. Hum Pathol 30: 158-67. [Crossref]
- Hsieh MS, Tseng YH, Hua SF, Chou YH (2015) Cystic alveolar adenoma: an unusual clinical presentation of a rare lung neoplasm. Pathology 47: 78-80. [Crossref]
- Petrella F, Rizzo S, Pelosi G, Borri A, Galetta D et al. (2010) Giant alveolar adenoma causing severe dyspnoea. J Thorac Oncol 5: 1088-1090. [Crossref]
