Morphological Characteristics of Aortic Stenosis in Familial Hypercholesterolemia and Non-Familial Hypercholesterolemia in the Elderly
A B S T R A C T
Background: Patients with familial hypercholesterolemia are known to have an extremely high risk of coronary artery disease owing to high levels of low-density lipoprotein-cholesterol since birth. In addition, aortic stenosis among patients with familial hypercholesterolemia has also been reported besides coronary disease. The aim of this study was to characterize the histopathological differences in excised aortic valves for aortic stenosis between patients with familial hypercholesterolemia and non-familial hypercholesterolemia.
Subjects and Methods: Six familial patients (3 homozygous, 3 heterozygous familial hypercholesterolemia patients), and 18 non-familial hypercholesterolemia patients underwent pathological and immunohistochemical examinations for aortic valves macroscopically and microscopically at aortic valve replacement surgery for stenosis.
Results: Our study revealed that calcification of aortic valves among homozygous hypercholesterolemia showed a much milder degree than those of non-familial patients. Moreover, the age at surgery for stenosis in the case of homozygotes was significantly less than that of heterozygous hypercholesterolemia and non-familial hypercholesterolemia patients. In addition, CD68-positive macrophages infiltrated the aorta side (fibrosa) in all familial hypercholesterolemia patients. However, the macrophage accumulation in the aortic valves of non-familial hypercholesterolemia patients was recognized in the middle layer (spongiosa) near calcification and left ventricular side (ventricularis) of the aortic valves.
Conclusion: Lipid is one of the important factors for aortic valve fibrosis and stenosis in hypercholesterolemia. This study suggested that the non-familial atherosclerotic aortic stenosis in the elderly is qualitatively different from aortic valves in familial hypercholesterolemia, primarily owing to calcification resulting from age and long-term degeneration and inflammatory responses.
Keywords
Aortic stenosis, familial hypercholesterolemia, calcific aortic stenosis, histology
Introduction
Most familial hypercholesterolemia (FH) belongs to an autosomal dominant disease caused by low-density lipoprotein (LDL) receptor gene mutations. Furthermore, to date, four other types of very rare causative genes for autosomal recessive hypercholesterolemia (ARH) have been identified: LDL receptor (LDLR) gene mutation (FH1); apolipoprotein B (APOB) gene mutation (FH2); and gain-of-function (GOF) mutation of proprotein convertase subtilisin/ kexin type 9 (PCSK9) gene (FH3) and abnormalities in the LDLR adapter protein 1 (LDLRAP1) gene. FH patients are known to have an extremely high risk of coronary artery disease (CAD) owing to high LDL-cholesterol (LDL-C) levels since birth. In addition, FH homozygotes show significantly high levels of LDL-C and total cholesterol (TC) at 500-900 mg/dL and 600-1,000 mg/dL, respectively. In addition to a high incidence rate of CAD, the frequency of aortic stenosis (AS) is very high.
Thickening, adhesion, and sclerosis of the valve cusp occur owing to prolonged inflammatory response, eventually leading to calcification and aortic valve stenosis. The causes of AS are divided into congenital bicuspid valve, rheumatic, and age-related degeneration. In recent years, with the increase in the aging population and the decline in rheumatic heart diseases, the number of AS cases, particularly among the elderly, which are caused by age-related degeneration and calcification of the aortic valve has increased [1]. It has been reported that advanced age is a strong risk factor for calcification in AS; in addition, hyperlipidemia, which is also a risk factor for arteriosclerosis and inflammatory responses (such as macrophage accumulation and oxidative stress reaction) are considered risk factors for AS [2-5]. A long-term large prospective cohort study in the Western countries has demonstrated that a genetic predisposition to elevated LDL-C is associated with aortic valve calcification and AS onset [6].
Aortic valve injury is known to occur within a relatively short period of time in homozygous FH (Ho-FH) patients and during the middle phase in heterozygous FH (He-FH) patients, whereas the damage occurs gradually over the years in non-FH patients. Extensive changes in the aortic root and aortic valve have been reported as characteristics of AS in Ho-FH patients; nonetheless, the pathological details of the aortic valve in patients with Ho-FH, He-FH, and age-related AS remain unclear [7]. Understanding these characteristics may prove important to treat the atherosclerotic lesions in the aorta and aortic valve and to control AS progression. In this study, we aimed to characterize the tissue component differences in AS between molecularly diagnosed Ho-HF, He-FH, and non-FH patients by examining the histopathology and immunopathology of their aortic valves.
Subjects and Methods
I Subjects
Three Ho-FH and three He-FH patients with DNA-based diagnosis of FH with confirmed LDL receptor mutation and 18 non-FH patients with high levels of plasma lipoprotein for long, who underwent aortic valve replacement and pathological examinations at the Department of Pathology in the National Cerebral and Cardiovascular Center between 2015 and 2019 were selected from approximately 100 cases registered at the Biobank [8, 9]. The diagnosis of FH was made in accordance with the “Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017” as Consensus Statement of Japan Atherosclerosis Society [10].
II Methods
i Echocardiographic Findings
AS evaluation was performed based on the peak velocity (PV) and aortic valve area (AVA) measurements obtained from the preoperative echocardiographic findings. (EPIQ 7C, Royal Philips, Amsterdam, the Netherlands).
ii Computed Tomography
CT of the aorta and heart were performed in some cases (Aquilion ONE, Canon Medical Systems, Tochigi, Japan). The degree of calcification was evaluated.
iii Pathological Findings
A Gross Pathology
The size and thickness of the aortic valves taken from the Ho-FH, He-FH, and non-FH atherosclerotic patients were estimated by photomacrographs just after surgery.
B Pathological Analysis by Routine Histology
The paraffin embedded-aortic valves of Ho-FH patients, He-FH patients, and non-FH patients were stained with hematoxylin and eosin, Masson’s Trichrome for fibrosis, and the Elastica van Gieson for elastic fiber. The degree of calcification amounts of aortic valves was scored as 0: none, 1: mild, 2: moderate, and 3: high. The degree of inflammatory cell infiltration was scored as 0: none, 1: mild, 2: moderate, and 3: high. The thickness of the aortic valve was measured by the imaging software Olympus CellSens (Tokyo, Japan).
C Immunohistochemistry
Immunohistochemical staining was performed on paraffin-embedded sections for each aortic valve specimen using the Bond Polymer Refine Detection Kit (Leica Biosystems, Germany) and monoclonal antibodies against apolipoprotein E (ApoE; Gene Tex, Irvine, USA) and the macrophage marker CD68 (Agilent Technologies, Santa Clara, CA, USA). A complex of rabbit anti-mouse IgG antibody and peroxidase-labeled anti-rabbit IgG antibody was formed with the primary antibody. The positive cells in the tissue sections showed dark brown staining owing to the reaction of 3,3’-diaminobenzidine and hydrogen peroxide, which was performed using an auto-staining system (Bond-III; Leica Biosystems). The stained tissue specimens were microscopically observed, and the area was calculated by Olympus CellSens.
iv Serum Lipid Levels
Plasma total cholesterol and lipoprotein levels were measured at the first examination before treatment. An automatic analyzer (LABOSPECT 008; Hitachi High-Tech Corporation, Tokyo, Japan) employing an enzymatic method (Sekisui Medical Co., Ltd., Tokyo, Japan) was utilized to determine the TC levels. LDL-C levels were calculated using the Friedewald equation (excluding TG levels of ≥400). The values obtained at the first outpatient visit were used.
v Statistical Analysis
Comparisons among groups were performed using the ANOVA test and Tukey's HSD test in continuous variable; the comparisons in categorical variables were performed using the Fisher's exact test, and a p-value of <0.05 was considered statistically significant.
Table 1: Clinical Characteristics of the AS patients with Hyperlipidemia.
|
Ho-FH(n=3) |
He-FH(n=3) |
non-FH(n=18) |
p-value(ANOVA test) |
|
|
Age (yrs) at AVR |
31±10 *, ** |
71±11 |
68±11 |
<0.001 |
|
Female (%) |
2 (66.7) |
2 (66.7) |
9 (50) |
NA |
|
AVA (cm2) |
0.9±0.1 |
0.8±0.2 |
1.0±0.6 |
0.821 |
|
peak velocity (m/sec) |
5.4±1.6 |
4.7±1.2 |
4.0±1.0 |
0.147 |
|
Hypertension (%) |
1 (33.3) |
3 (100) |
12 (66.7) |
0.304 |
|
Diabetes mellitus (%) |
0 |
3 (100) |
10 (55.6) |
0.060 |
|
CAD (%) |
3 (100) |
3(100) |
8 (44) |
0.083 |
|
TC (mg/dL) pre-mediation |
734±180 *, ** |
266±58 |
227±45 |
<0.001 |
|
TC (mg/dL) at AVR |
260±28 *, ** |
151±10 |
200±25 |
0.016 |
|
LDL-C (mg/dL) pre-medication |
546 (n=1) |
186±50 |
127±35 |
NA |
|
LDL-C (mg/dL) at AVR |
200±2 *, ** |
94±19 |
123±22 |
0.016 |
AS: aortic stenosis; AVA: aortic valve area, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol.
*p<0.05 versus He-FH, **p<0.05 versus non-FH, based on the Tukey's HSD (honestly significant difference) test.
“TC and LDL-C pre-medication” showing the data before apheresis and medication in Ho-FH and the data in He- and non-FH at initial diagnosis before induction of statin.
Results
I Age at Aortic Valve Replacement
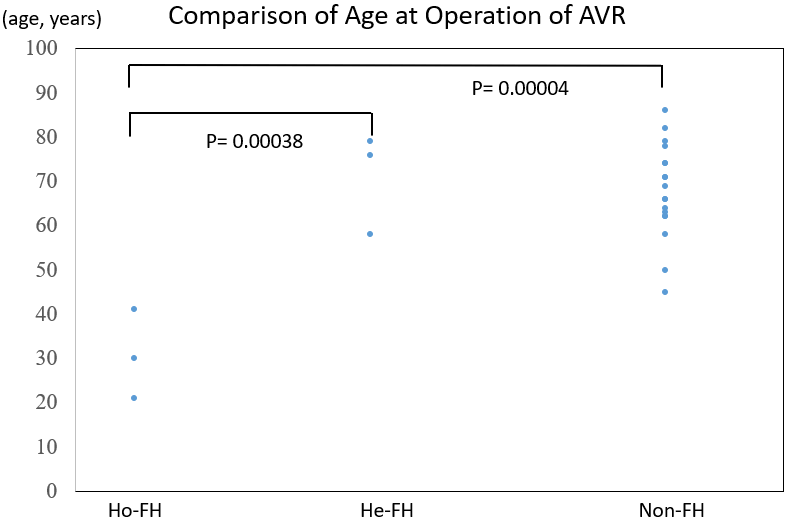
Table 1 shows the characteristics of Ho-FH, He-FH, and non-FH patients who were pathologically examined just after aortic valve replacement (AVR). The age at surgery was 31 ± 10 years for Ho-FH patients, 71 ± 11 years for He-FH patients, and 68 ± 11 years for non-FH patients. The age at surgery of Ho-FH patients was significantly younger than those of the other two groups of patients (Figure 1). All the Ho- and He-FH patients presented with CAD, whereas only 44% of the non-FH patients presented with this complication. In terms of risk factors, 1 (33%) Ho-FH patient, 3 (100%) He-FH patients, and 12 (67%) non-FH patients presented with hypertension; moreover, 3 (100%) He-FH patients and 10 (56%) non-FH patients had diabetes.
Figure 1: Comparison of the ages at aortic valve replacement for AS of each group of patients with homozygous familial hypercholesterolemia (Ho-FH), heterozygous familial hypercholesterolemia (He-FH), and non-familial hypercholesterolemia (Non-FH).
AVR: Aortic valve replacement, Ho-FH: Homozygous Familial Hypercholesterolemia, He-FH: Heterozygous Familial Hypercholesterolemia, Non-FH: non-Familial Hypercholesterolemia.
II Comparison of AS Lesions between FH and Non-FH Patients
i Echocardiographic Findings
Based on the preoperative echocardiographic findings, AVA was 0.9 ± 0.1 cm2 in Ho-FH patients, 0.8 ± 0.2 cm2 in He-FH patients, and 1.0 ± 0.6 cm2 in non-FH patients. The PV was 5.4 ± 1.6 m/s in Ho-FH patients, 4.7 ± 1.2 m/s in He-FH patients, and 4.0 ± 1.0 m/s in non-FH patients. The AS was severe in the Ho- and He-FH patients and moderate to severe in the non-FH patients (Table 1).
ii Serum Lipid Levels
At the first visit to our hospital, the TC levels induction were 734 ± 180, 266 ± 58, and 227 ± 45 mg/dl in the Ho-FH, He-FH patients, and non-FH patients, respectively; likewise, the LDL-C levels were 546 ± 60, 186 ± 50, and 127 ± 35 mg/dl, respectively, in the three groups of patients (Table 1). All He-FH patients took statin treatment before AVR. Serum lipid levels became lower at operation in the Ho-FH patients, followed by the He-FH patients and non-FH patients.
iii Comparison of Aortic Valve Calcium by Computed Tomography (CT)
CT of one female case of Ho-FH showed aortic valvular thickening without obvious calcification with supravalvular aortic atherosclerosis [9]. A female He-FH case showed calcium deposition of aortic and mitral valves and coronary arteries. CT revealed massive calcification in all non-FH cases.
Table 2: Histological Calcific Score (HCS) of Aortic Valves.
|
Ho-FH(n=3) |
He-FH(n=3) |
non-FH(n=18) |
p-value(ANOVA test) |
|
|
HCS |
0.6*, ** |
3.0 |
2.5±0.6 |
<0.001 |
|
Severity of calcification. Calc area of aortic valve >40% |
0 |
3 (100%) |
10 (56%) |
HCS: Histological Calcification Score. 0; ; calc area <5%, non, 1; calc area <15%, 2; calc area 15 <40%, 3; calc area more than 40%.
*p<0.001 versus He-FH, based on the Tukey's HSD test.
**p<0.001 versus non-FH, based on the Tukey's HSD test.
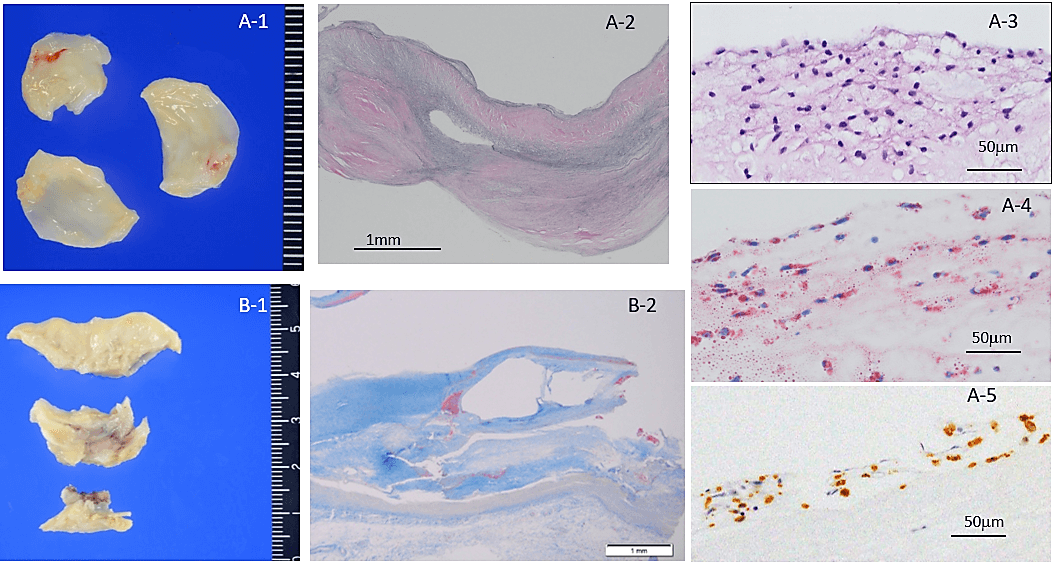
Figure 2: A) Representative excised aortic valve in a 41-year-old homozygous FH female. A-1) macroscopic appearance of fibrous thickening all cusps. A-2) Fibrous thickened cusp without calcification, EVG stain. A-3) foamy macrophage accumulation of aorta side (fibrosa) of cusp, H&E stain. A-4) Oil red O stain showing lipid in macrophages in fibrosa. A-5) CD68 positive macrophages in fibrosa. B-1) Supravalvular atherosclerosis of Valsalva sinus. B-2) Photomicrograph of B-2 showing atherosclerosis with calcification, Masson’s trichrome stain.
iv Comparison of Pathological Findings of Aortic Valves of Ho-FH, He-FH, and Non-FH
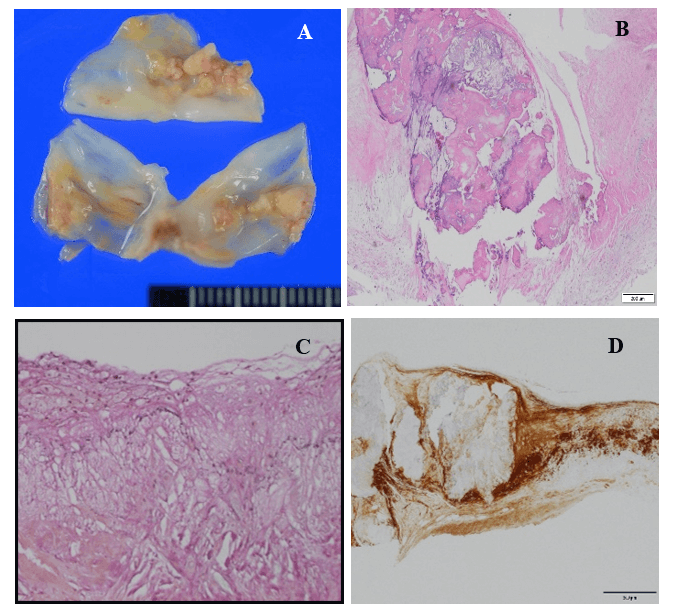
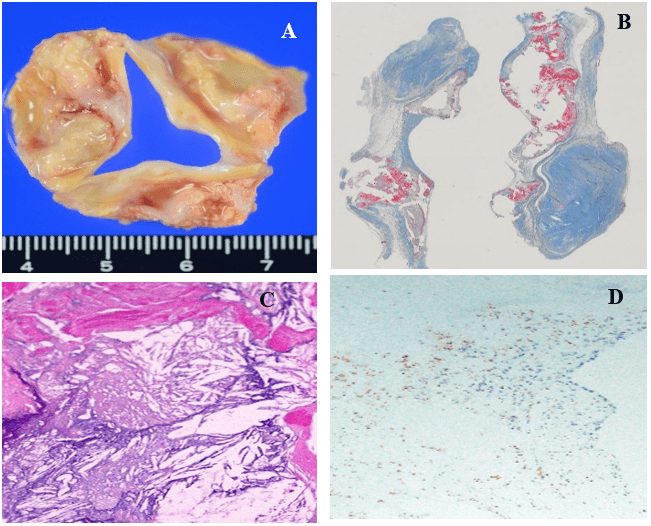
The macroscopic and microscopic degree of calcification amount of excised aortic valve (mean score) was 0.6 in the Ho-FH patients, 3.0 in the He-FH patients, and 2.5 in the non-FH patients. The ratios of severe calcific deposition were 0%, 100%, and 56% in the Ho-FH, He-FH, and non-FH patients (Table 2). Yellow fibrosclerotic lesions without macroscopic calcification were clearly seen in the aortic valve of the Ho-FH patients (Figure 2). Histopathological and immunohistochemical findings showed accumulation of foamy macrophages containing lipid in the aorta side layer, so-called fibrosa (Figures 2A-3, 2A-4 & 2A-5). In addition, the high level of lipid deposition in the sinus of Valsalva was observed in the Ho-FH patients (Figures 2B-1 & 2B-2). He-FH cases showed massive deposition of ApoE around calcified nodules (Figure 3). There were foamy macrophage and cholesterol crystal accumulation on the aorta side (fibrosa) of aortic valves in FH patients, whereas calcification and atherosclerosis were mainly observed in the middle layer (spongiosa) in the non-FH patients and on the left ventricular side (ventricularis) in almost half of non-FH patients (Figure 4).
Figure 3: Representative excised aortic valve in a 58-year-old heterozygous FH male. A) macroscopic appearance of yellowish nodular calcification from aorta side. B) Nodular calcification of Figure 3A, H&E stain. C) foamy macrophage and cholesterol crystal accumulation of aorta side of cusp, H&E stain. D) ApoE immunohistochemistry showing diffuse deposit Apo E in aortic valve.
Figure 4: Representative excised aortic valve in a 69-year-old non-FH male. A) macroscopic stenosis with massive nodular calcification of aortic valve. B) Nodular calcification, Masson’s trichrome stain. C) Cholesterol crystal accumulation with calcification of cusp, H&E stain. D) CD68 positive macrophage accumulation around nodular calcification not fibrosa, immunohistochemistry for CD68 macrophages.
Discussion
Because of the presence of hyperlipidemia since a young age, patients with FH have a higher risk of developing coronary artery disease and aortic valve stenosis compared to atherosclerosis as lifestyle-related illness of the elderly that develops over time.
In this study, all Ho- and He-FH patients had CAD, whereas only 44% of the non-FH patients presented with this complication. The age at surgery for AS among the Ho-FH patients was remarkably younger than those of the other groups; however, the age of the He-FH patients did not significantly differ from that of the non-FH patients. Histopathologically, aortic valves developing AS in patients with Ho-FH had thickened fibrosis with very mild calcification. On the other hand, aortic valves in the cases of He-FH develop the complication owing to effects of other arteriosclerosis risk factors, such as hypertension and diabetes [10]. In the non-FH patients, AS develops over a long period of time owing to the effects of elevated serum lipid levels and other arteriosclerosis risk factors [11]. Extensive arteriosclerosis in the sinus of Valsalva along with supravalvular AS was observed in all the Ho-FH patients, but not in He-FH and non-FH patients. Ho-FH patients often develop AS and supravalvular AS at high frequencies (41%) [12, 13]. In contrast, the frequency has been reported to be low in He-FH patients [14]. Although some cases of double heterozygous mutations and compound heterozygous mutations, which carry more than two types of gene mutations, present with severe pathologies, He-FH shows mild to severe symptoms. Supravalvular AS may be observed in some patients with severe FH. Similar findings were observed in the current study, thereby indicating that high cumulative cholesterol levels since birth are associated with the progression of arteriosclerosis and aortic valve lesions in Ho-FH patients.
In terms of the correlation between serum lipid levels and aortic valve calcific lesions, particularly in non-FH patients, some patients presented with aortic valve calcifications despite high TC and LDL-C levels, whereas others had extremely high aortic valve calcifications despite low TC levels and LDL-C levels. These findings suggested that both TC and LDL-C levels did not have any strong effects on the aortic valve calcification. However, the Cardiovascular Health Study (CHS), which investigated the presence or absence of aortic valve calcification and related factors, reported that age, male sex, history of smoking, history of hypertension, lipoprotein (a) [Lp(a)] levels, and LDL-C levels were significant risk factors for aortic valve calcification [15, 16]. Furthermore, a genetic risk score (GRS)-based genetic increase in LDL-C levels has been reported to be associated with calcification and AS onset [6]. In addition, calcified lesions tend to progress when LDL-C levels increase in patients with AS [14]. These results differ from those of the present study; however, this could be because the target patients were different.
The pathological analysis of the aortic valves revealed lipid deposition, macrophage and T-cell infiltration, basement membrane destruction of elastic fibers, fibrosis, and calcification in all patients. The calcification amount was quite low in Ho-FH, but other histological evidence such as valve thickening or fibrosis showed no differences in the extent of these changes were observed among the Ho-FH, He-FH, and non-FH patients.
Immunochemical staining revealed high ApoE deposition in all patients, with no significant differences among the three groups. Conversely, significant differences in CD68 positive macrophages were observed among the three groups. Macrophages were observed on the aorta side of aortic valve in all the Ho-FH and He-FH patients, whereas CD68 was mostly deposited in the middle layer around atherosclerosis and calcification and left ventricular side of the aortic valve in the non-FH patients, which may have directly enhanced the calcification of the aortic valve.
In patients with Ho-FH, atherosclerosis of the ascending aorta causing supra-valvular stenosis was stronger, and both Apo B deposition as well as foam cell accumulation were reported in the aorta and aortic valve [7]. During atherosclerosis of the aorta, monocytes enter the arterial intima via chemotactic factors present within the area of the lesion, form macrophages, and ingest the accumulated LDLs. In the aortic valve, the macrophages ingest the LDLs attached to the valve.
In patients with age-related AS, shear stress can cause endothelial cell injury in the valve, leading to lipid deposition, accumulation of inflammatory cells such as macrophages and T-lymphocytes, and the migration and proliferation of fibroblasts and smooth muscle cells causing fibrosis in the valve [2, 15]. The fibroblasts in the valve differentiate into osteoblasts and are involved in calcium deposition [17]. In addition, it has been reported that vascular smooth muscle cell apoptosis plays an important role in calcification in the blood vessels in patients with AS; this process is thought to be similar to the progression of arteriosclerosis in the blood vessels [2, 3, 18, 19-21].
Furthermore, oxidized LDLs are localized and are involved in the progression of the AS lesions, and these oxidized LDLs have been demonstrated to promote the migration and proliferation of smooth muscle cells and fibroblasts [19, 22]. Therefore, it is believed that macrophage activation and the action of oxidized LDLs in AS may promote the migration and proliferation of fibroblasts and smooth muscle cells in the valve and can be involved in the progression of aortic valve fibrous thickening. Moreover, it has been reported that interstitial cells in the human aortic valve, Lp (a) increases the activity of alkaline phosphatase, a calcification-related enzyme, and the levels of phosphate and calcium; it also induces aortic valve apoptosis and calcification [23]. In this study, macrophage accumulation was confirmed in the aortic valve of all the AS patients, strongly indicating that it plays an important role in the progression of AS.
The pathological examination of the lesions of aortic valve showed that in FH, the deposition of CD68, which is highly noted in macrophages, was observed on the aorta side and fibrous thickening without severe calcification. Thus, the LDLs that were attached to the aortic side that bends during aortic valve opening may have penetrated the valve with the macrophages in the patients with FH. Consequently, the direct involvement of oxidized LDLs might have contributed to the progression of aortic valve lesions. However, although CD68 deposition was observed on the aortic side in He-FH patients, as noted in homozygous FH patients, the age of AS onset was delayed. This delay may be related to the concentration of LDL in the blood. Furthermore, because the cumulative cholesterol levels since birth were higher in FH patients than in non-FH patients, the LDLs could be strongly involved in the progression of lesions. The degree of pathology among the He-FH patients differs depending on the type of genetic mutation; moreover, in some severe cases, the age of onset may be early. Conversely, in non-FH patients, the age of AS onset was similar to that in He-FH patients, but CD68 positive macrophages were observed in the middle layer mostly around calcification and atherosclerosis and left ventricular side of the aortic valve. Thus, in addition to the action of the LDLs and macrophages, aging and other risk factors, as well as hemodynamic stress may be strongly involved in the progression of AS over time.
One of the main limitations of this study is that the conditions of the early lesions were unknown before aortic valve replacement because the tissue specimens were collected at the time of operation for AS.
Limitations of the Study
The main limitation of this study is the small number of Ho-FH and He-FH patients because of very rare diseases in the single-center study. Statistical analysis is limited to Tukey’s HSD because of small numbers. Most of the patients with He-FH and non-FH in this study had been treated with statins for a long period. This may reflect the relatively lower cholesterol levels than those of Ho-FH.
Conclusion
The direct involvement of high-level LDLs in FH was considered to be an important factor for the development of AS owing to the fibrous thickening, not calcification of the aortic valve and supra-valvular atherosclerosis that develops from younger ages in patients with FH. On the other hand, this study suggested that calcification and degeneration of the aortic valve in age-related AS qualitatively different from FH.
Ethical Approval
This study was approved by the Research Ethics Committee of the National Cerebral and Cardiovascular Center (R19085).
Consent
Informed consent to participate in the study was obtained from the patients registered at NCVC Biobank.
Acknowledgements
We would like to thank NCVC Biobank staff as well as Mrs. Arata and Ms. Katayama as Pathology Laboratory technologists for their cooperation in completing this study.
Conflicts of Interest
None.
Funding
None.
Author Contributions
Concept and Design: N.O., T.F. and H.I-U.; Data Collection and Analysis: N.O., T.T., M.N. M.H. and H.I-U.; Supervision: M.H-S. and H.I-U.; Critical Review: M.H-S.
Article Info
Article Type
Research ArticlePublication history
Received: Tue 17, Mar 2020Accepted: Sat 04, Apr 2020
Published: Mon 13, Apr 2020
Copyright
© 2023 Hatsue Ishibashi-Ueda. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JICOA.2020.02.03
Author Info
Hatsue Ishibashi-Ueda Mariko Harada-Shiba Michio Noguchi Mika Hori Naotaka Ohta Tomoyuki Fujita Tsutomu Tomita
Corresponding Author
Hatsue Ishibashi-UedaDepartment of Pathology, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan
Figures & Tables
Table 1: Clinical Characteristics of the AS patients with Hyperlipidemia.
|
Ho-FH(n=3) |
He-FH(n=3) |
non-FH(n=18) |
p-value(ANOVA test) |
|
|
Age (yrs) at AVR |
31±10 *, ** |
71±11 |
68±11 |
<0.001 |
|
Female (%) |
2 (66.7) |
2 (66.7) |
9 (50) |
NA |
|
AVA (cm2) |
0.9±0.1 |
0.8±0.2 |
1.0±0.6 |
0.821 |
|
peak velocity (m/sec) |
5.4±1.6 |
4.7±1.2 |
4.0±1.0 |
0.147 |
|
Hypertension (%) |
1 (33.3) |
3 (100) |
12 (66.7) |
0.304 |
|
Diabetes mellitus (%) |
0 |
3 (100) |
10 (55.6) |
0.060 |
|
CAD (%) |
3 (100) |
3(100) |
8 (44) |
0.083 |
|
TC (mg/dL) pre-mediation |
734±180 *, ** |
266±58 |
227±45 |
<0.001 |
|
TC (mg/dL) at AVR |
260±28 *, ** |
151±10 |
200±25 |
0.016 |
|
LDL-C (mg/dL) pre-medication |
546 (n=1) |
186±50 |
127±35 |
NA |
|
LDL-C (mg/dL) at AVR |
200±2 *, ** |
94±19 |
123±22 |
0.016 |
AS: aortic stenosis; AVA: aortic valve area, TC: total cholesterol, LDL-C: low-density lipoprotein cholesterol.
*p<0.05 versus He-FH, **p<0.05 versus non-FH, based on the Tukey's HSD (honestly significant difference) test.
“TC and LDL-C pre-medication” showing the data before apheresis and medication in Ho-FH and the data in He- and non-FH at initial diagnosis before induction of statin.
Table 2: Histological Calcific Score (HCS) of Aortic Valves.
|
Ho-FH(n=3) |
He-FH(n=3) |
non-FH(n=18) |
p-value(ANOVA test) |
|
|
HCS |
0.6*, ** |
3.0 |
2.5±0.6 |
<0.001 |
|
Severity of calcification. Calc area of aortic valve >40% |
0 |
3 (100%) |
10 (56%) |
HCS: Histological Calcification Score. 0; ; calc area <5%, non, 1; calc area <15%, 2; calc area 15 <40%, 3; calc area more than 40%.
*p<0.001 versus He-FH, based on the Tukey's HSD test.
**p<0.001 versus non-FH, based on the Tukey's HSD test.
AVR: Aortic valve replacement, Ho-FH: Homozygous Familial Hypercholesterolemia, He-FH: Heterozygous Familial Hypercholesterolemia, Non-FH: non-Familial Hypercholesterolemia.
References
- Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS (1999) Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341: 142-147. [Crossref]
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD (1994) Characterization of the early lesion of ‘degenerative valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 90: 844-853. [Crossref]
- Olsson M, Dalsgaard CJ, Haegerstrand A, Rosenqvist M, Rydén L et al. (1994) Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol 23: 1162-1170. [Crossref]
- Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J Jr et al. (2008) Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol 28: 463-470. [Crossref]
- Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña Silva R et al. (2008) Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52: 843-850. [Crossref]
- Smith JG, Luk K, Schulz CA, Engert JC, Do R et al. (2014) Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 312: 1764-1771. [Crossref]
- Yutani C, Go S, Imakita M, Ishibashi Ueda H, Hatanaka K et al. (1987) Autopsy findings in two patients with homozygous familial hypercholesterolemia. Special references to apolipoprotein B localization and internalization defect of low density lipoprotein. Acta Pathol Jpn 37: 1489-1504. [Crossref]
- Hori M, Ohta N, Takahashi A, Masuda H, Isoda R et al. (2019) Impact of LDLR and PCSK9 pathogenic variants in Japanese heterozygous familial hypercholesterolemia patients. Atherosclerosis 289: 101-108. [Crossref]
- Kakuta T, Fujita T, Fukushima S, Kawamoto N, Matsumoto Y et al. (2018) Two Cases of Surgical Management of Supravalvular Aortic Stenosis in Familial Hypercholesterolemia. Ann Thorac Surg 105: e171-e174. [Crossref]
- Harada Shiba M, Arai H, Ishigaki Y, Ishibashi S, Okamura T et al. (2018) Guidelines for Diagnosis and Treatment of Familial Hypercholesterolemia 2017. J Atheroscler Thromb 25: 751-770. [Crossref]
- Kawaguchi A, Yutani C, Yamamoto A (2003) Hypercholesterolemic valvulopathy: an aspect of malignant atherosclerosis. Ther Apher Dial 7: 439-443. [Crossref]
- Brook GJ, Keidar S, Boulos M, Grenadier E, Wiener A et al. (1989) Familial homozygous hypercholesterolemia: clinical and cardiovascular feature in 18 patients. Clin Cardiol 12: 333-338. [Crossref]
- Summers RM, Andrasko Bourgeois J, Feuerstein IM, Hill SC, Jones EC et al. (1998) Evaluation of the aortic root by MRI: insights from patients with homozygous familial hypercholesterolemia. Circulation 98: 509-518. [Crossref]
- Rallidis L, Naoumova RP, Thompson GR, Nihoyannopoulos P (1998) Extent and severity of atherosclerotic involvement of the aortic valve and root in familial hypercholesterolemia. Heart 80: 583-590. [Crossref]
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS et al. (1997) Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol 29: 630 -634. [Crossref]
- Pohle K, Maffert R, Ropers D, Moshage W, Stilianakis N et al. (2001) Progression of aortic valve calcification. Circulation 104: 1927-1932. [Crossref]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J et al. (2003) Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181-2184. [Crossref]
- Freeman RV, Otto CM (2005) Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111: 3316-3326. [Crossref]
- Hsu HH, Camacho NP (1999) Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis 143: 353-362. [Crossref]
- Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM et al. (2000) Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 87: 1055-1062. [Crossref]
- Olsson M, Thyberg J, Nilsson J (1999) Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 19: 1218-1222. [Crossref]
- Stiko Rahm A, Hultgårdh Nilsson A, Regnström J, Hamsten A, Nilsson J (1992) Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb 12: 1099-1109. [Crossref]
- Yu B, Hafiane A, Thanassoulis G, Ott L, Filwood N et al. (2017) Lipoprotein(a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci 2: 358-371. [Crossref]
- Thanassoulis G, Cambell CY, Owens DS, Smith JG, Smith AV et al. (2013) Genetic association with valvular calcification and aortic stenosis. N Eng J Med 368: 503-512. [Crossref]
