Microfractured Adipose Tissue Graft for the Advanced Treatment of Non-Healing Cutaneous Fistulas
A B S T R A C T
Chronic cutaneous non-healing fistulas very often are dehiscences of surgical or traumatic wounds that do not repair properly and progressively undergo intrafistular and perifistular fibrosis. The fibrous tissue constitutes a natural barrier to the progression of the fistula repair process and represents the major cause of non-healing and chronicization even in a context of proper vascularization. The microfractured autologous adipose graft allows to provide the tissues involved in the fibrotic process with a regenerative stimulus by MSCs (mesenchymal stem cells) contained in adipose clusters of 0.3 mm. In this context, MSCs are able to secrete cytokines with antibiotic, antifibrotic, angiogenic and analgesic effects. The micro-fragmentation technique guarantees a high regenerative effect, as the MSCs are not isolated enzymatically with the simultaneous destruction of the adipose tissue. The micro-fragmentation allows the maintainance of the structure of the adipose cluster including microvessels and allows it to amplify by 6000 times the active surface that exposes the MSCs. Our experience with the mechanical microfracturing method of lipoaspirate consists of 41 treatments. In 7 cases the graft was performed due to the presence of a non-healing cutaneous fistula, which lasted from 128 to 243 days. In 6 cases we achieved immediate repair and closure of the fistula while in one case the procedure failed. The purpose of the paper is to describe in detail our experience by an accurate description of the implemented method of the used device accompanied by the document with adequate photographic documentation.
Keywords
Cutaneous fistula, tracheotomic fistula, adipose graft, Lipogems®, non-healing fistula
Introduction
The technique of autologous adipose tissue transplantation has been widely used since the 1980s both for purely aesthetic purposes in association with surgical lifting and for reconstructive ones. Autologous adipose tissue transplantation has been applied mainly for volume restoration in the breast or maxillofacial field. The regenerative action has been exploited both for the treatment of radiodermatitis and for the treatment of burns and retracting scar pathology. The main problem of the treatments is represented by the unpredictability of the results and of the regenerative effect that varies according to the rate of resorption of the grafted tissue that occurs in the postoperative weeks. The choice of an effective method, easily achievable and the standardization of processes allows to obtain consistent and comparable results.
Adipose tissue represents the largest organ of the organism and has multiple structural and endocrine functions. The rich microvascular network and especially the perivascular cellular component actively participate in tissue regenerative and reparative processes. Indeed, pericytes are richly present in adipose tissue and represent the precursors of Mesenchymal Stem Cells (MSCs) [1].
The fundamental three dimensional structure for the functions described above is the adipose niche which consists of a native microenviroment within tissue that contains adipocytes and other cells embedded in a collagen scaffold with a vascular network. The components of the adipose niche consist of adipocytes, extracellular matrix (collagen and connective tissue), pericytes (wrapped around capillaries), preadipocytes, microvasculature and other cell types (Table 1).
Table 1:
|
Pericytes |
CD140b+, CD146+, NG2+,
CD31-, CD34-, CD144-, vWF- |
|
Adipocytes |
|
|
Adipose Derived Stem Cells |
CD13+, CD29+, CD34+/-,
CD44+, CD90+, CD104a+, CD14-, CD31-, CD45-, CD106-, CD144-, CD146-, Asma- |
|
Pre-Adipocytes |
|
|
Endothelial & Progenitor
Cells |
CD31+, CD34+, CD90+, CD146+,
VWF+, CD45- |
|
Haematopoetic Cells |
Monocytes/Macrophages |
Bone marrow has always been considered the progenitor of hematopoietic cells and was the first regenerative tissue to be studied, however adipose tissue provides greater regenerative effect as it is abundant, easy to harvest, and possesses more MSCs [2]. The concentration of MSCs in bone marrow aspirate is approximately 20,000/cc, while in microfragmented lipoaspirate the concentration is 100,000/cc. This figure is calculated by underestimation because many MSCs remain trapped in fibroadiposis clusters and therefore can’t be counted [3].
The importance of MSCs lies in their secretome, which is the set of proteins that are released into the extracellular space. The secretome of MSCs includes exosomes, proteins and RNAs (non-coding RNAs). Exosomes are sufficiently small vesicles that they can penetrate the cell membrane and deliver biochemical messages. Exosomes were discovered in the 80's and were initially considered as simple cellular waste or residues; only recently the enormous potential of these nanospheres, with a diameter of less than 150nm whose surface consists of a lipid membrane, emerged as these actually carry important surface proteins and mRNAs, such as to consider MSCs as real Medicine Signaling Cells [4].
The tissue trauma and consequently of the wall of microvessels, involves the immediate release of pericytes in the interstitium and their transformation into MSCs, with the consequent release of cytokines activating macrophages in an anti-inflammatory and immunomodulatory sense. In the following days, cytokines with antibiotic, antifibrotic (HGF, bFGF, KGF, SDF-1, MIP-1a, MIP-1b), angiogenetic (VEGF, IGF-1, bFGF, IL-6, TGFβ1) and analgesic actions are produced [5, 6].
In cases where enzymatic processing of the liposuction aspirate is performed, the total destruction of the adipose tissue is obtained by extracting a cell concentrate called Stromal Vascular Factor (SVF) which contains about 500 times more cells than bone marrow aspirate [3]. However, enzymatic processing is a GMP procedure, i.e. a Good Manufacturing Practice procedure that from the regulatory point of view can only be performed in specialized laboratories. It should also be considered that there is a poor correlation between the number of MSCs in the transplanted tissue and its biological and clinical action [3]. In fact, there is no direct correlation between MSCsm quantity of secretomes and exosomes and final biological action [7, 8].
Adipose tissue microfragmentation is not a GMP method, as it does not aim at destroying adipose tissue and releasing isolated MSCs into a cell mix such as SVF. Microfragmentation is a gentle mechanical method to obtain microclusters of adipocytes rich in surface intact microvessels surrounded by pericytes. Microfragmentation is a procedure that, starting from 3 mm adipose clusters present in the original lipoaspirate, allows to wash and fragment the lipoaspirate until obtaining a MFAT (Micro Fractured Adipose Tissue) with 0.3/0.5 mm clusters completely preserved and intact. This type of fragmentation allows to amplify by about 6000 times the total surface of intact and pericyte rich microvessels compared to the original lipoaspirate [9-12]. MFAT is structurally identical to the original lipoaspirate but with greater biological properties such that it can be considered a natural implantable bioreactor [13].
Materials and Methods
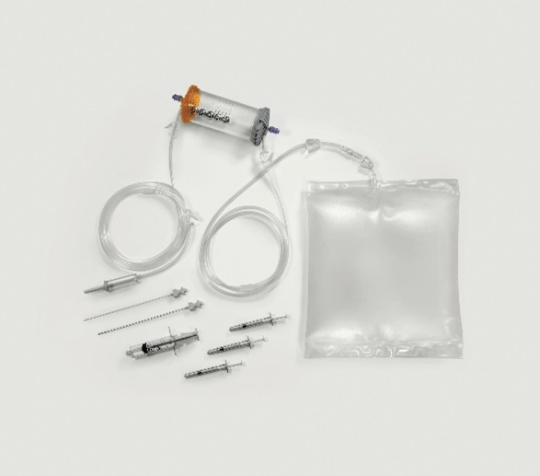
Adipose tissue is aspirated using 20 cc VacLock syringes and through disposable aspirating cannulas (13G) with multiple 2x1 mm oval holes. The size and geometry of the holes has been designed to ensure a rapid collection, which does not damage the lipoaspirate and allows to obtain a tissue consisting of adipose clusters of about 3 mm in diameter.
In the second phase of the procedure, the lipoaspirate is inserted into a completely closed device (Figure 1) that allows to wash the adipose tissue eliminating blood and inflammatory residues. The passage through two sharp progressive filters allows with only hydraulic action to gradually fragment the tissue until obtaining clusters of 0.3 mm in diameter. The possibility to carry out the fragmentation in a closed system and in the absence of air allows to avoid oxidative processes and to avoid the destruction of adipocytes by bursting as they are constantly kept immersed in saline solution even when subjected to pressure and hydraulic thrust aimed at the transit through the two progressive filters.
Figure 1: Complete kit for MFAT processing.
Inside the device there are five steel spheres that allow a better debridement and cleaning of the adipose tissue from the oily and hematic emulsion and to guarantee a greater dispersion of the clusters that allows an easier and less traumatic passage of the washed lipoaspirate through the second filter. The correct procedure involves shaking the device for no less than 20 seconds and no more than 90 seconds. Adipose tissue is pushed by hydraulic force through the second filter which passes directly into a 10 cc syringe. Its re-injection into recipient tissues is accomplished through the use of a 1 mL luer lock syringe and a 20G needle.
The treatment of the fistula includes first a phase in which an accurate curettage of the fistulous passage, washing and disinfection is performed. The next step involves filling the fistula with MFAT and closing the fistulous orifices with a non-resorbable stitch. Subsequently, a perifistular MFAT infiltration is performed to obtain an ab extrinsic compression of the fistulous passageway itself. Dressing is performed strictly without compression. Intraoperative prophylactic antibiotic treatment with cefazolin and during the first four postoperative days with amoxicillin and clavulanic acid is administered.
Results
From 2015 to 2021, we have performed microfragmented autologous adipose tissue transplantation in 41 patients, for treatment of retracting scars, breast or facial profile improvements following reconstructive surgery, improvement of anal sphincter incontinence, and treatment of diabetic or venous insufficiency ulcers.
In 7 cases, treatment was given for non-healing skin fistulas. Five cases were female patients and 2 cases were male. The mean age was 57.2 years (32-82). The anatomic site was in one case at the neck (Figures 2-4), in one case at the level of the thigh (Figures 5 & 6), in one case at the arm (Figures 7-11), in two cases in the interdigital spaces of the foot (Figures 12-14), and in two cases at the level of the foot (dorsum of the foot and forefoot) (Figures 15-17).
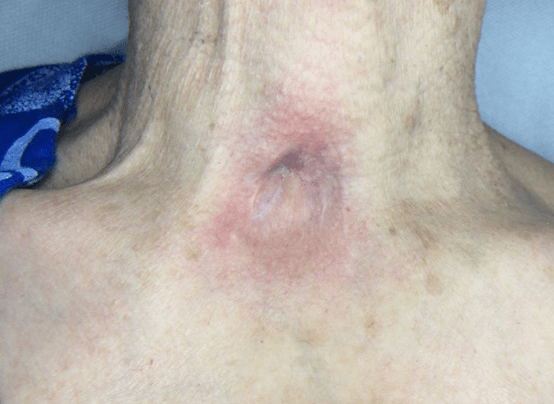
Figure 2: Case no. 1: Neck fistula in previous tracheotomy (181 days).
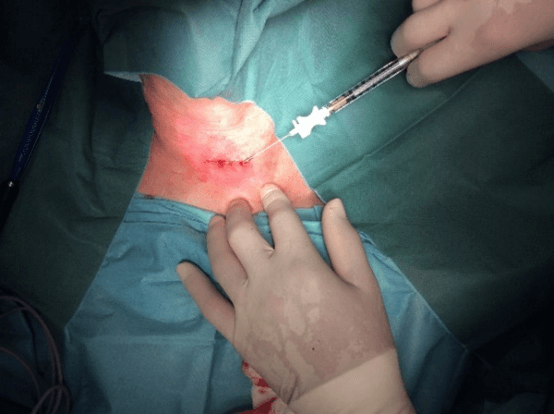
Figure 3: Case no. 1: Intraoperative image, intra and peri fistular infiltration of MFAT after debridement and suturing of the orifices.
Figure 4: Case no. 1: Repaired fistula.
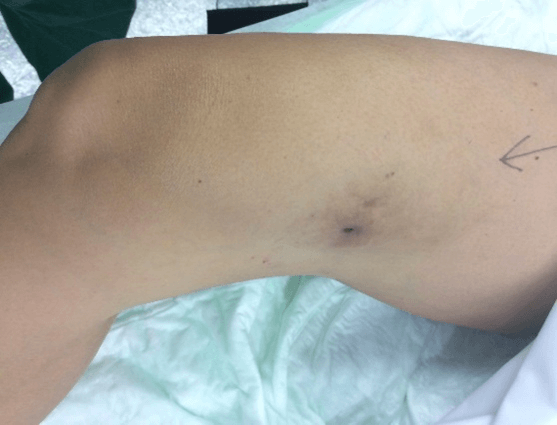
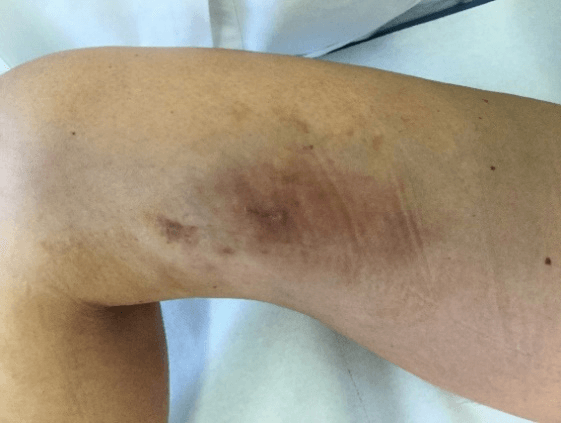
Figure 5: Case no. 2: Fistula in the right thigh following an infected hematoma (133 days).
Figure 6: Case no. 2: Repaired fistula.
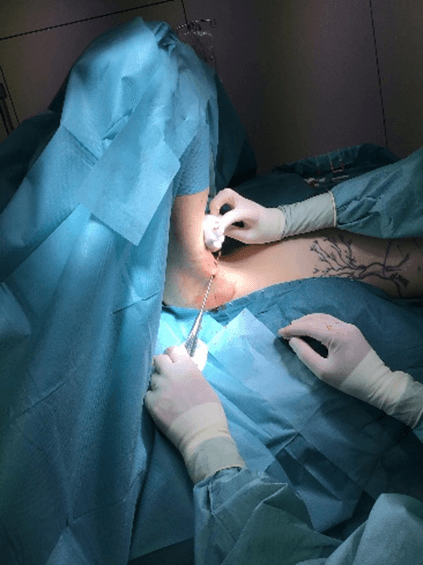
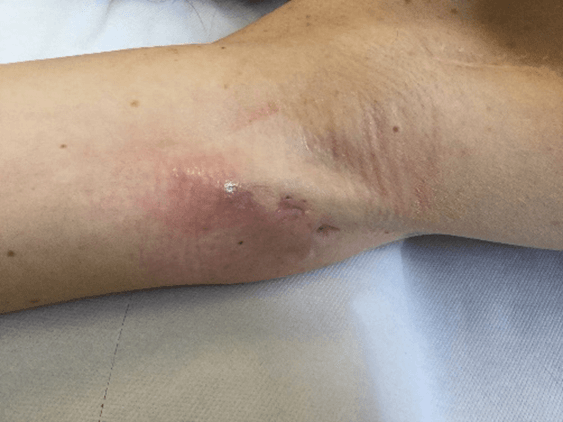
Figure 7: Case no. 3: Axillary fistula following a dog bite in patient previously subjected to lymphadenectomy (150 days).
Figure 8: Case no. 3: Axillary fistula: intraoperative debriding.
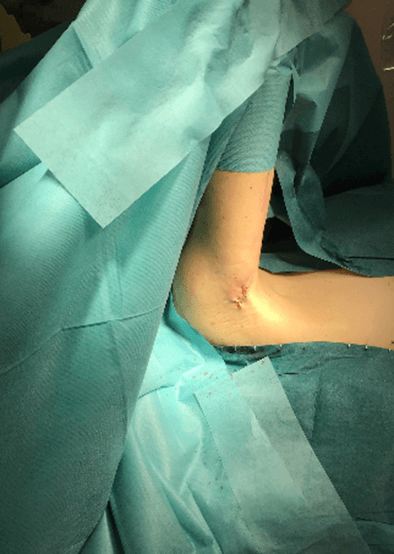
Figure 9: Case no. 3: Axillary fistula: suture.
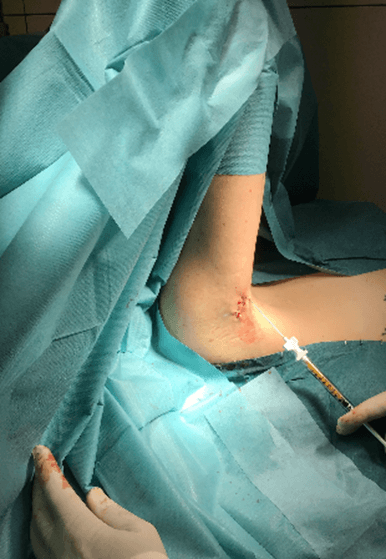
Figure 10: Case no. 3: Axillary fistula, infiltration of intra and peri fistular MFAT.
Figure 11: Case no. 3: Healed axillary fistula.
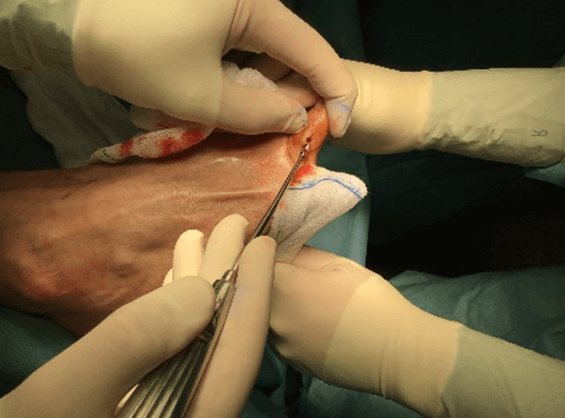
Figure 12: Case no. 4: Interdigital fistula, intraoperative debriding.
Figure 13: Case no. 4: Interdigital fistula, MFAT infiltration.
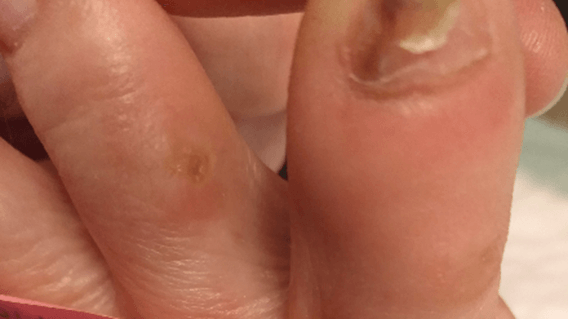
Figure 14: Case no. 4: Interdigital fistula healed.
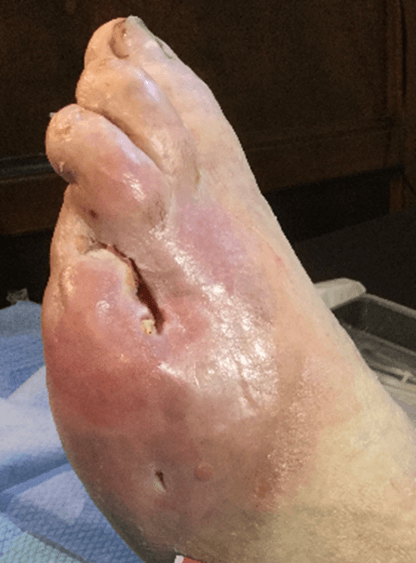
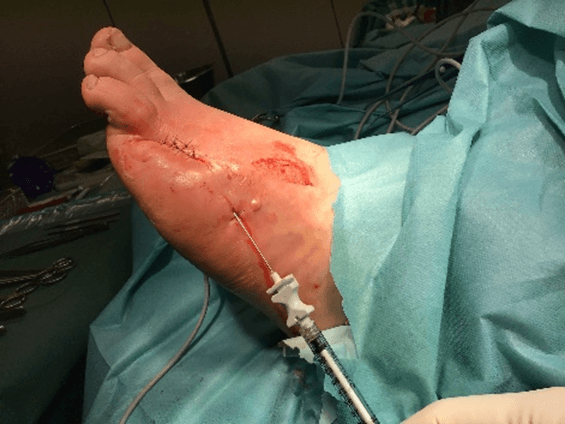
Figure 15: Case no. 6: Dorsal foot fistula in DFU after 4th and 5th finger amputation (128 days).
Figure 16: Case no. 6: Dorsal foot fistula in DFU, intra and peri lesional infiltration of MFAT after debridement and suturing of the fistula orifices.
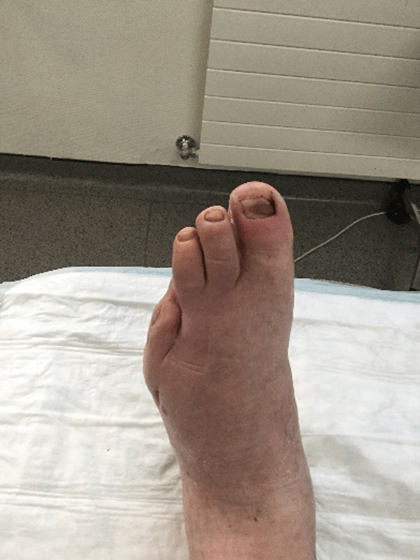
Figure 17: Case no. 6: DFU dorsal foot fistula healed.
Fistulas persisted for between 128 and 243 days with a mean of 165 days. Comorbidities included diabetes, hypertension, arteriopathy, COPD, AF, conditions with steroid treatment, and sleep apnea syndromes, interestingly, there was a case of a lymphatic fistula in the arm following a dog bite in a patient who had previously undergone lymphadenectomy for treatment of melanoma of the upper extremity. In 6 cases we obtained an immediate postoperative healing while in one case we obtained a total failure probably due to an incorrect indication on diabetic foot severely arteriopathic. In the three cases in which there was an aesthetic implication (neck lesion for inveterate tracheostomy fistula, arm and thigh lesions) we obtained a clear aesthetic improvement with satisfaction of the patients. An overview of the case history can be seen in (Table 2).
Discussion
The MFAT is a tissue washed of oily impurities, blood residues or dead cells. It consists of an emulsion of adipose microclusters perfectly intact from a structural and anatomical point of view, with a maximum diameter of 300 microns (0.3 mm). Unlike SVF transplantation in which enzymatically isolated cells were transplanted and eventually added to centrifuged adipose tissue, in the case of local injection of MFAT there is to all intents and purposes an implantation of tissue micrografts. In fact, the transferred microclusters possess an intact anatomical structure with adipose and microvascular scaffolds supporting pericytes and MSCs. Growth factors and cytokines are produced by exosomes and in a tissue specific manner depending on the tissue and environment in which the MFAT is implanted. Small size and anatomical integrity are factors favouring extensive engraftment of transplanted tissue.
The reparative process of a tissue has been shown to be strongly favoured by tissue microvascular density, which varies inversely with age. General conditions and comorbidities such as diabetes are factors that promote an early decrease in tissue vascular microdensity [14, 15].
The reduction of the diameter of the clusters from 3 mm to 0.3 mm leads to a significant increase of the units obtaining from a single cluster about 1000 microclusters perfectly intact and covered on the surface by capillaries. The surface itself is increased up to about 6000 times, thus exposing a large amount of capillaries and consequently of pericytes ready to function in a completely natural way.
It has been demonstrated both in vitro and in recipient tissues that MFAT actively induces microcapillary development through a mechanism of neovasculogenesis [16]. One of the main mechanisms by which MFAT enhances tissue healing capacity is to stably increase vascular density resulting in true tissue rejuvenation [15, 17, 18]. In the orthopaedic field, there is extensive experience with intra and periarticular treatments with a clear superiority of MFAT in inducing articular cartilage regeneration over enzymatically obtained SVF or expanded MSCs in culture [19-21].
In the field of vulnology, there are many publications demonstrating the efficacy of MFAT in the treatment of dehiscences from minor amputations in the diabetic foot, in pressure ulcers following orthosis; in our paper we wanted to report the efficacy of MFAT in the treatment of difficult-to-heal skin fistulas of different etiologies [22, 23].
Table
2:
|
Case |
Age |
Site |
Type
of fistula |
Comorbidity |
Results |
Donor
site |
Lipoaspirate
amount |
MFAT
infiltration amount |
Persistence
of the fistula (days) |
|
1 |
80 |
NECK |
Post tracheostomy
fistula for Guillain Barrè syndrome |
COPD, atrial
fibrillation, pace maker, anticoagulation therapy |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
60
cc |
10
cc |
181 |
|
2 |
54 |
THIGH |
Post-hematoma
fistula of the thigh |
Factor VIII
deficiency thrombophilic syndrome |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
220
cc |
53
cc |
133 |
|
3 |
32 |
ARM |
Post dog bite
lymphatic fistula in previous axillary lymphadenectomy for melanoma |
Previous melanoma
in immunotherapy treatment, previous venous thrombosis of the upper limb in
anticoagulant treatment, asthma in steroid treatment |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
100
cc |
25
cc |
150 |
|
4 |
60 |
FINGER |
Interdigital
fistula |
Underweight
patient, recent spinal surgery with prolonged immobilization |
Complete
immediate healing of the fistula and no aesthetic implication |
Right inner thigh
and right hip |
40
cc |
3
cc |
173 |
|
5 |
82 |
FINGER |
Interdigital
fistula |
Overweight,
arterial hypertension, ischemic heart disease, arterial disease undergoing
angioplasty, non-insulin dependent diabetes mellitus, rheumatoid arthritis
under steroid treatment. |
Complete
immediate healing of the fistula and no aesthetic implication |
Lower
abdomen |
¨40
cc |
3
cc |
147 |
|
6 |
57 |
FOOT |
Post surgical
fistula for DFU (diabetic foot ulcer) |
Overweight,
arterial hypertension, COPD, sleep apnea syndrome, non-insulin dependent
diabetes mellitus, paroxysmal atrial fibrillation on anticoagulant treatment |
Complete
immediate healing of the fistula and no aesthetic implication |
Abdomen
hips |
200
cc |
40
cc |
128 |
|
7 |
81 |
FOOT |
Post surgical
fistula in arteriopathic patients |
Arteriopathy,
insulin dependent diabetes mellitus, valvular heart disease, pacemaker, COPD,
sleep apnea syndrome |
Failed |
Abdomen
hips |
120
cc |
30
cc |
243 |
Conclusion
Adipose tissue is the ideal source for the recruitment of mesenchymal stem cells both because it is easily accessible, because it is generally present in abundance and because it is extremely rich in pericytes that are progenitors of MSCs. The microfragmentation involves an optimization of the liposuction, increasing enormously the biological efficacy through the creation of micrografts structurally intact, easy to take root and expressing a biologically active surface amplified up to 6000 times. The method used is well standardized, completely mechanical, in a closed system and with a constant protection of the aspirated tissues guaranteed by the constant immersion in the saline solution in the absence of air that ensures functional structural and biological integrity of MFAT.
Our experience is anecdotal because in our case we are not able to carry out prospective studies both for ethical reasons and for the exiguity of the numbers, however the rigor with which the interventions have been carried out and data and iconographic documentation have been preserved we can affirm that the treatment of non-healing cutaneous fistulas by MFAT transplantation allows in many cases an immediate repair or an acceleration of the repair process provided that the surgical indication is correct.
Author Contributions
M.DM. Conceptualization, formal analysis, data curation; L.DP. Writing—review and editing; K.G. Supervision, validation.
Funding
None.
Informed Consent
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
None.
Article Info
Article Type
Research ArticlePublication history
Received: Thu 22, Sep 2022Accepted: Wed 12, Oct 2022
Published: Fri 28, Oct 2022
Copyright
© 2023 Marco De Monti. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.RGM.2022.02.05
Author Info
Marco De Monti Laura De Pellegrin Ken Galetti
Corresponding Author
Marco De MontiEOC - Beata Vergine Regional Hospital, Department of Surgery, Mendrisio, Switzerland
Figures & Tables
Table 1:
|
Pericytes |
CD140b+, CD146+, NG2+,
CD31-, CD34-, CD144-, vWF- |
|
Adipocytes |
|
|
Adipose Derived Stem Cells |
CD13+, CD29+, CD34+/-,
CD44+, CD90+, CD104a+, CD14-, CD31-, CD45-, CD106-, CD144-, CD146-, Asma- |
|
Pre-Adipocytes |
|
|
Endothelial & Progenitor
Cells |
CD31+, CD34+, CD90+, CD146+,
VWF+, CD45- |
|
Haematopoetic Cells |
Monocytes/Macrophages |
Table
2:
|
Case |
Age |
Site |
Type
of fistula |
Comorbidity |
Results |
Donor
site |
Lipoaspirate
amount |
MFAT
infiltration amount |
Persistence
of the fistula (days) |
|
1 |
80 |
NECK |
Post tracheostomy
fistula for Guillain Barrè syndrome |
COPD, atrial
fibrillation, pace maker, anticoagulation therapy |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
60
cc |
10
cc |
181 |
|
2 |
54 |
THIGH |
Post-hematoma
fistula of the thigh |
Factor VIII
deficiency thrombophilic syndrome |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
220
cc |
53
cc |
133 |
|
3 |
32 |
ARM |
Post dog bite
lymphatic fistula in previous axillary lymphadenectomy for melanoma |
Previous melanoma
in immunotherapy treatment, previous venous thrombosis of the upper limb in
anticoagulant treatment, asthma in steroid treatment |
Complete
immediate healing of the fistula and aesthetic improvement |
Abdomen
hips |
100
cc |
25
cc |
150 |
|
4 |
60 |
FINGER |
Interdigital
fistula |
Underweight
patient, recent spinal surgery with prolonged immobilization |
Complete
immediate healing of the fistula and no aesthetic implication |
Right inner thigh
and right hip |
40
cc |
3
cc |
173 |
|
5 |
82 |
FINGER |
Interdigital
fistula |
Overweight,
arterial hypertension, ischemic heart disease, arterial disease undergoing
angioplasty, non-insulin dependent diabetes mellitus, rheumatoid arthritis
under steroid treatment. |
Complete
immediate healing of the fistula and no aesthetic implication |
Lower
abdomen |
¨40
cc |
3
cc |
147 |
|
6 |
57 |
FOOT |
Post surgical
fistula for DFU (diabetic foot ulcer) |
Overweight,
arterial hypertension, COPD, sleep apnea syndrome, non-insulin dependent
diabetes mellitus, paroxysmal atrial fibrillation on anticoagulant treatment |
Complete
immediate healing of the fistula and no aesthetic implication |
Abdomen
hips |
200
cc |
40
cc |
128 |
|
7 |
81 |
FOOT |
Post surgical
fistula in arteriopathic patients |
Arteriopathy,
insulin dependent diabetes mellitus, valvular heart disease, pacemaker, COPD,
sleep apnea syndrome |
Failed |
Abdomen
hips |
120
cc |
30
cc |
243 |



References
1. Tremolada C,
Palmieri G, Ricordi C (2010) Adipocyte transplantation and stem cells: plastic
surgery meets regenerative medicine. Cell Transplant 19: 1217-1223. [Crossref]
2. Mattar P, Bieback K
(2015) Comparing the Immunomodulatory Properties of Bone Marrow, Adipose
Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front Immunol
6: 560. [Crossref]
3. Mautner K, Bowers
R, Easley K, Fausel Z, Robinson R (2019) Functional Outcomes Following
Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate
Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med 8:
1149-1156. [Crossref]
4. Caplan AI, Correa D
(2011) PDGF in bone formation and regeneration: new insights into a novel
mechanism involving MSCs. J Orthop Res 29: 1795-1803. [Crossref]
5. Caplan AI, Correa D
(2011) The MSC: an injury drugstore. Cell Stem Cell 9: 11-15. [Crossref]
6. Diaz AC, Gomez JMQ,
Dorado G (2020) Extracellular Vesicles Derived From Mesenchymal Stem Cells
(MSCs) in Regenerative Medicine: Applications in Skin Wound Healing. Front
Bioeng Biotechnol 8: 146. [Crossref]
7. Xu T, Yu X, Yang Q,
Liu X, Fang J et al. (2019) Autologous Micro-Fragmented Adipose Tissue as Stem
Cell-Based Natural Scaffold for Cartilage Defect Repair. Cell Transplant 28:
1709-1720. [Crossref]
8. Vezzani B, Shaw I,
Lesme H, Yong L, Khan N et al. (2018) Higher Pericyte Content and Secretory
Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically
Derived Stromal Vascular Fraction. Stem cells Transl Med 7: 876-886. [Crossref]
9. Copcu HE, Oztan S
(2021) Not Stromal Vascular Fraction (SVF) or Nanofat, but Total Stromal-Cells
(TOST): A New Definition. Systemic Review of Mechanical Stromal-Cell Extraction
Techniques. Tissue Eng Regen Med 18: 25-36. [Crossref]
10. Aronowitz JA,
Lockhart RA, Hakakian CS (2015) Mechanical versus enzymatic isolation of
stromal vascular fraction cells from adipose tissue. Springerplus 4:
713. [Crossref]
11. Trivisonno A,
Alexander RW, Baldari S, Cohen SR, Rocco GD et al. (2019) Intraoperative
Strategies for Minimal Manipulation of Autologous Adipose Tissue for Cell-and
Tissue-Based Therapies: Concise Review. Stem cells Transl Med 8:
1265-1271. [Crossref]
12. Tremolada C,
Colombo V, Ventura C (2016) Adipose Tissue and Mesenchymal Stem Cells: State of
the Art and Lipogems® Technology Development. Curr Stem Cell Rep 2:
304-312. [Crossref]
13. Tremolada C (2022)
Microfractured Adipose Tissue Grafts (Lipogems) and Regenerative Surgery. J
Orthop Res Ther 7: 1210.
14. Bouglé A, Rocheteau
P, Briand D, Hardy D, Verdonk F et al. (2019) Beneficial role of
adipose-derived mesenchymal stem cells from microfragmented fat in a murine
model of duchenne muscular dystrophy. Muscle Nerve 60: 328-335. [Crossref]
15. Mastrangelo AN,
Haus BM, Vavken P, Palmer MP, Machan JT et al. (2010) Immature animals have
higher cellular density in the healing anterior cruciate ligament than
adolescent or adult animals. J Orthop Res 28: 1100-1106. [Crossref]
16. Bianchi F, Maioli
M, Leonardi E, Olivi E, Pasquinelli G et al. (2013) A new nonenzymatic method
and device to obtain a fat tissue derivative highly enriched in pericyte-like
elements by mild mechanical forces from human lipoaspirates. Cell Transplant
22: 2063-2077. [Crossref]
17. Casarotti GA,
Chiodera P, Tremolada C (2018) Menopause: new frontiers in the treatment of
urogenital atrophy. Eur Rev Med Pharmacol Sci 22: 567-574. [Crossref]
18. Tremolada C,
Allegri M, Slevin M (2019) Mesenchymal Stromal Cells and Micro Fragmented
Adipose Tissue: New Horizons of Effectiveness of Lipogems. J Stem Cell Res
Dev Ther 5: 017.
19. Mikkelsen RK, Blønd
L, Hölmich LR, Mølgaard C, Troelsen A et al. (2021) Treatment of osteoarthritis
with autologous, micro-fragmented adipose tissue: a study protocol for a
randomized controlled trial. Trials 22: 748. [Crossref]
20. Desando G,
Bartolotti I, Martini L, Giavaresi G, Aldini NN et al. (2019) Regenerative
features of adipose tissue for osteoarthritis treatment in a rabbit model:
enzymatic digestion versus mechanical disruption. Int J Mol Sci 20:
2636. [Crossref]
21. Filardo G, Tschon
M, Perdisa F, Brogini S, Cavallo C et al. (2022) Micro-fragmentation is a valid
alternative to cell expansion and enzymatic digestion of adipose tissue for the
treatment of knee osteoarthritis: A comparative preclinical study. Knee Surg
Sports Traumatol Arthrosc 30: 773-781. [Crossref]
22. Lonardi R, Leone N, Gennai S, Borsari GT, Covic T et al. (2019) Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: a randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther 10: 223. [Crossref]
23. Copeland R, Martin J (2021) Chronic prosthesis-related residual limb ulcer treated with autologous micro-fragmented adipose tissue. Regen Ther 18: 21-23. [Crossref]
