Journals
Metastasis of Adult Granulosa Cell Tumor to Mediastinum, Lung, and Pleura 11 and 25 Years after Initial Diagnosis, Report of Two Cases and Review of the Literature
A B S T R A C T
Ovarian adult granulosa cell tumors are relatively rare low-grade malignant tumor. Metastasis of granulosa cell tumors often happens a long interval after initial surgical resections. This could lead to diagnostic difficulties due to loss of clinical follow up and lack of detailed clinical history. We present 2 cases of metastatic granulosa cell tumor to the lung, pleura, and mediastinum 11 and 25 years respectively after resection of primary tumors. The first case was a 67-year-old woman presented with a large mediastinal mass who underwent surgical resection with a clinical impression of thymoma. The tumor was largely necrotic, and focal viable tumor was composed of epithelioid cells with pale eosinophilic cytoplasm, irregular nuclei, and rare grooved nuclei. No typical Call-Exner body was identified. The histological differential diagnosis was broad, and a metastatic tumor was highly suspected. Additional clinical information was requested; it revealed a history of left ovarian mass with a diagnosis of adult granulosa tumor 11 years ago. The mediastinal tumor was confirmed to be adult granulosa cell tumor by immunohistochemistry. The second case involves a 74-year-old woman who presented with left pleural effusion and a large subpleural lung mass, with biopsy demonstrating classic histological features of adult granulosa cell tumor. She had bilateral salpingo-oophorectomy 25 years ago, so diagnostic details of the ovarian lesion were unavailable. Adult granulosa cell tumor can present as late metastasis at unusual locations. The possibility of metastatic granulosa tumors should always be kept in mind, and clinical history is critical to promote an accurate diagnosis of late onset metastatic granulosa cell tumor.
Introduction
Ovarian granulosa cell tumor has two subtypes, juvenile and adult, both characterized by indolent growth. Adult granulosa cell tumors account for more than 90% of all granulosa cell tumors. It is typically a low-grade malignant tumor and presents as stage I disease with unilateral ovarian involvement. Unfortunately, a small proportion of the stage I cases will subsequently recur, typically 4-7 years from the initial diagnosis, leading to death in 50% of these patients [1]. Most of the recurrences occur in preserved genital tract structures. The liver is the most common extra-pelvic metastatic site followed by the intestine [2]. Other distant metastasis has been reported at many extra-abdominal sites including lung, brain, bone, and supraclavicular lymph node [3-5]. Furthermore, there have been relatively few case reports with very late recurrences, occurring at least 10 years after initial surgical resections [2, 6-7]. For example, Hasiakos et al reported a recurrence of granulosa cell tumor 20 years after initial diagnosis [6]. Due to prolonged interval between the original diagnosis and metastasis, the patient herself may not remember the nature of the primary disease, and the relevant past medical history may not be readily available. It could pose a difficulty for clinicians to make an accurate diagnosis of metastatic adult granulosa cell tumors. We describe here 2 cases of metastatic adult granulosa cell tumors: one case involving the mediastinum and another case involving the pleura and lung, 11 and 25 years after initial diagnosis.
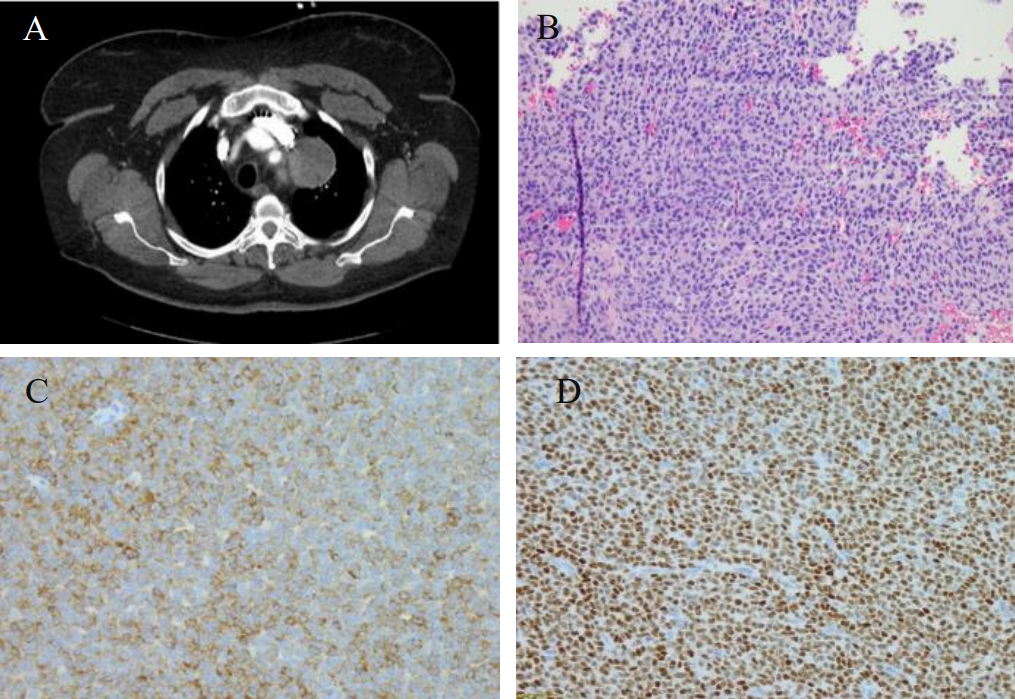
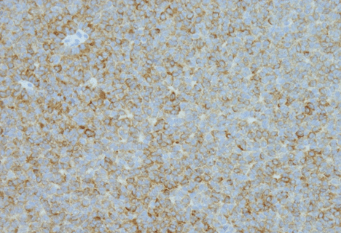
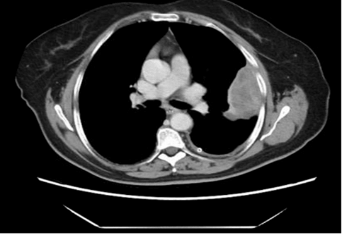
Figure 1: A. CT showed a round mildly enhancing mass within the superior middle mediastinum, measures 4.8 x 5.2 x 6.4 cm. There is a thin fat plane separates this mass from the subjacent thoracic aorta and this mass is seen abutting the great vessels without affecting patency; B. Hematoxylin and eosin stain; C. Inhibin immunostain; D. WT-1 immunostain.
Case 1
The patient, a 67-year-old with past medical history of asthma, hypertension, and cerebral aneurysm, presented to a primary care physician with left-sided chest wall pain in December 2017. Her past medical history was significant for abdominal hysterectomy and salpingo-oophorectomy at an outside hospital 11 years ago, but detailed pathologic diagnostic information was not available. The patient’s chest pain was dull in characteristic without aggravating factors. She had no cough, fever, chills or shortness of breath. Her routine laboratory investigations were within normal limits with no evidence of leukocytosis. The patient underwent a chest X-ray which showed opacity in the area of the aortic arch that raised concern for a possible aortic aneurysm. Subsequent CT angiogram was negative for aortic aneurysm. However, an encapsulated, round 6.4 x 5.2 x 4.8 cm mildly enhancing mass was noted in the superior portion of the middle mediastinum. There was a thin fat plane separating this mass from the subjacent thoracic aorta. The mass was seen abutting the great vessels without affecting vascular patency (Figure 1A). A PET scan was also performed and demonstrated PET-avidity of the periphery of the mediastinal mass with left pleural effusion. Given its location and appearance on imaging, this mass was concerning for a possible thymoma. The patient underwent a robotic resection of the mediastinal mass. Macroscopically, the lesion was well circumscribed and encapsulated with a gray-tan lobular cut surface. Focal hemorrhage and necrosis were present. Histology showed a well encapsulated tumor with areas of infarction and necrosis. Viable tumor was present at the periphery of the lesion, which was composed of epithelioid cells with pale eosinophilic cytoplasm, irregular nuclei and rare nuclear inclusions. Nuclear grooves were present but rare. Tumor cells formed small nests, trabeculae, and abortive tubular structures. No typical Call-Exner body was identified (Figure 1B). Given broad histological differential diagnosis, a panel of immunohistochemical stains was performed to further characterize the tumor. Tumor cells were strong diffusely positive for estrogen receptor (ER), progesterone receptor (PR), CD56, inhibin, calretinin, and CD99; focally positive for pan-keratins (AE1/AE3 and CK8/18) and PAX8; and completely negative for EMA, Ber-EP4, TTF-1 and GATA3 (Figure 1C and 1D). The tumor morphology and immunoprofile were compatible with a metastatic adult granulosa cell tumor, Additional clinical information was requested from the outside hospital which revealed a history of bilateral salpingo-oophorectomy and hysterectomy that was performed 11 years ago. The outside hospital pathology report described a 19 cm right ovarain mass. Histologic examination revealed an adult granulosa cell tumor without involvement of the left ovary or other organs at the time of surgery. The final diagnosis of the mediastinal mass is metastatic adult granulosa cell tumor.
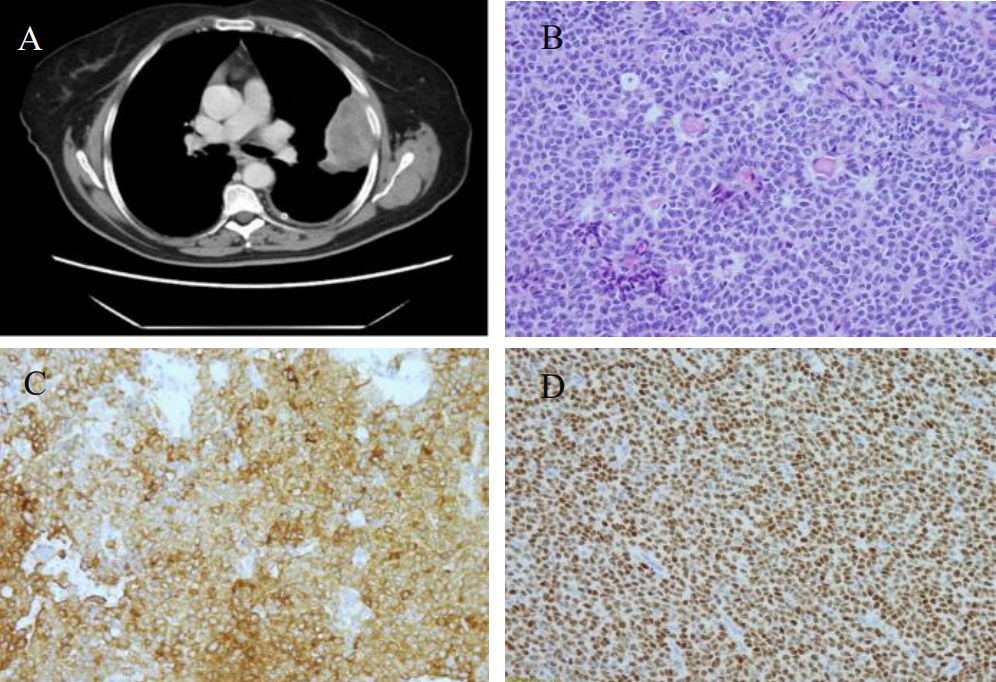
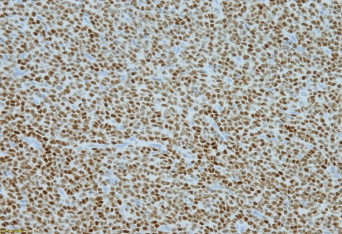
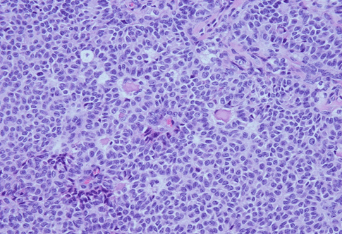
Figure 2: A. CT showed a large heterogeneous subpleural enhancing solid mass involving the left upper lobe, measuring approximately 7.9 x 3.8 cm and left pleural effusion. B. Hematoxylin and eosin stain; C. Inhibin immunostain; D. WT-1 immunostain.
Case 2
A 74-year-old Caucasian woman with past medical history of diabetes and hereditary angioedema presented to the oncology department due to shortness of breath and left pleural effusion in March 2018. Her past surgical history was significant for bilateral salpingo-oophorectomy in 1993, which was diagnosed as ovarian “benign” cysts as stated by the patient. The patient denied chest pain, cough, fever, and chills. Chest X-ray and Computed Tomography (CT) were performed and revealed left pleural effusion and a large heterogeneous subpleural solid mass involving the left upper lobe, measuring approximately 7.9 x 3.8 cm. In addition, three separate pulmonary nodules were identified: one located in the left upper lobe measuring 8 mm, one in the left lower lobe measuring 7 mm, and the third one in the right upper lobe measuring 6 mm (Figure 2A). Two thoracocenteses were subsequently performed, which were interpreted as positive for malignancy with unknown primary. A pleural core biopsy was performed for further evaluation. The biopsy demonstrated a tumor with diffuse, cord, trabecular, and insular growth patterns with Call-Exner body like structures. Tumor cells were intermediate in size with scant cytoplasm. Most tumor cell nuclei were elongated with nuclear grooves (Figure 2B). Immunohistochemical stains were performed to further characterize the tumor. Tumor cells were positive for WT1, inhibin and CD99, negative for calretinin, CD10, TTF-1, and a broad spectrum of keratins (Figure 2C and 2D). The histological features and immunostaining profile were consistent with metastatic adult granulosa cell tumor.
Discussion
The majority of patients with stage I adult granulosa cell tumors have a good prognosis. Only a small percentage of such tumors metastasize, which might pose diagnostic problem because of long interval between initial surgery and metastasis. Most recurrences in patients with adult granulosa cell tumors are intra-abdominal, whereas lung, pleura and mediastinal metastases are extremely rare [8]. Bryk et al followed 164 cases of molecularly defined adult granulosa cell tumors with a median follow-up time of 15.5 years. It showed 32% patients developed tumor recurrence with only 2 cases of lung metastasis (1.2%). While the intervals between initial diagnosis and lung metastasis for these two patients were not mentioned in the report [9]. The longest reported interval of lung metastasis from initial diagnosis of ovarian granulosa cell tumor was 36 years [10]. Pleural metastasis and exfoliation of tumor cells in pleural fluid is even rare and has only been described in 2 case reports to the best of our knowledge [10-11]. The case reported by Gupta et al presented as a stage IV granulosa cell tumor with exfoliation of tumor cells in both pleural and ascetic fluid with exudative effusions [11]. It has been speculated that pleural effusion occurs as a result of the passage of ascitic fluid through the diaphragmatic lymphatics, or through congenital defects. In one of our patients, the pleural effusion was associated lung metastasis extending to visceral pleura, which is similar to the one described by Kimura and colleagues [11]. Mediastinal metastasis of granulosa cell tumors is also extremely rare. Only three prior cases have been reported in the literature so far with one case having metastasis in the mediastinum 40 years after treatment of initial ovarian neoplasm [12-14]. Given the rarity of the tumor, late metastasis following initial diagnosis, and diverse histological features, diagnosis of metastatic adult granulosa cell tumor can be challenging, particularly if with a small core biopsy and unusual location. In the lung and pleura, the leading differential diagnosis includes reactive epithelial cells, neuroendocrine tumor, lymphoma, melanoma, primary adenocarcinoma, mesothelioma, and metastatic carcinoma such as thyroid carcinoma; whereas differential diagnosis for a mediastinal mass is broad and includes thymoma, lymphoma, germ cell tumor, intrathoracic thyroid, parathyroid lesion, and metastatic tumors including melanoma [12, 15].
Adult granulosa cell tumors can grow in a wide variety of patterns including sheet, cord, trabeculae, undulating ribbons, and nests. Call-Exner bodies are pathognomic, featuring microfollicles composed of granulosa cells surrounding a small space containing eosinophilic secretion. The tumor cells usually have scanty cytoplasm and angular to oval, often grooved nuclei. These histological features are diagnostically helpful if present. But exceptions to the classic patterns described above are not uncommon which might pose diagnostic difficulty not only for metastasis but primary tumors as well. Granulosa cells might contain bizarre enlarged hyperchromatic nuclei, multinucleated giant cells, and/or abundant eosinophilic or vacuolated cytoplasm. Rarely, the neoplastic granulosa cells display signet ring cell or spindle cell morphologies, even sarcomatous transformation with less conspicuous nuclear grooves [16]. It is extremely important to have proper clinical history when unusual histology causes troubles in distinction from other primary or metastatic tumors. Immunohistochemical stains are helpful in problematic tumors. Granulosa cell tumors are usually positive for inhibin, calretinin, WT1, steroidogenic factor-1, CD99, and CD56. The tumors could be positive for broad spectrum of keratin particularly low molecular weight keratins (CK8 and CK18) but typically negative for CK7 and EMA. Recent studies have shown that immunohistochemistry of transcription factor FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. It has higher sensitivity for the diagnosis of sex cord stromal tumors than α-inhibin and calretinin. FOXL2 staining was typically more intense in positive cases compared with either α-inhibin or calretinin [17]. Furthermore, greater than 90% adult granulosa cell tumors bear FOXL2 gene mutations, therefore, molecular studies could be used as an ancillary study in diagnostically challenging cases [18].
In conclusion, adult granulosa cell tumors can present as a late recurrence/metastasis long after initial surgical resection of the primary tumors. The patient may not remember the nature of the primary ovarian tumor because of remote history and being lost to follow up after the surgery. The diagnosis of tumor metastasis could be challenging. Histological findings and immunohistochemistry are essential for accurate pathology interpretation. However, clinical history and the possibility of very late recurrence and metastasis after initial surgical resection must be kept in mind to avoid delayed diagnosis.
Conflicts of Interest
None
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Sun 05, May 2019Accepted: Wed 22, May 2019
Published: Thu 06, Jun 2019
Copyright
© 2023 Xiuzhen Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.03.04
Author Info
Shaun Boyes Yihong Ma sara Javidiparsijani Vijayalakshmi Ananthanarayanan Xianzhong Ding Xiuzhen Duan
Corresponding Author
Xiuzhen DuanDepartment of Pathology, Loyola University Medical Center, Maywood, IL
Figures & Tables

References
- Färkkilä A, Haltia UM, Tapper J, McConechy MK, Huntsman DG et al. (2017) Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary. Ann Med 49: 435-447. [Crossref]
- Lee YK, Park NH, Kim JW, Song YS, Kang SB et al. (2008) Characteristics of recurrence in adult-type granulose cell tumor. Int J Gynecol Cancer 18: 642-647. [Crossref]
- Dubuc-Lissoir J, Berthiaume MJ, Boubez G, Van Nguyen T, Allaire G (2001) Bone metastasis from a granulosa cell tumor of the ovary. Gynecol Oncol 83: 400-404. [Crossref]
- Puri S, Mohindroo N, Mohindroo S, Sharma S (2015) Ovarian granulosa cell tumor metastatic too supraclavicular lymph node after 15 years of diagnosis: a case report. J Cytol 32: 213-214. [Crossref]
- Williams RJ, Kamel HM, Jilaihawi AN, Prakash D (2001) Metastatic granulosa cell tumour of the diaphragm 15 years after the primary neoplasm. Eur J Cardiothorac Surg 19: 516-518. [Crossref]
- Hasiakos D, Papakonstantinou K, Karvouni E, Fotiou S (2008) Recurrence of granulosa cell tumor 25 years after initial diagnosis. Eur J Gynaecol Oncol 29: 86-88. [Crossref]
- Givalos N, Liakakos T, Machairas A, Gakiopoulou H, Karatzas G (2005) Sequential recurrences of ovarian granulose cell tumor 10 and 11 years after initial diagnosis as haemoperitoneum and subhepatic mass: a case report and review of the literature. Eur J Gynaecol Oncol 26: 572-576. [Crossref]
- Schumer ST, Cannistra SA (2003) Granulosa cell tumor of the ovary. J Clin Oncol 21: 1180-1189.
- Bryk S, Färkkilä A, Bützow R, Leminen A, Tapper J et al. (2016) Characteristics and outcome of recurrence in molecularly defined adult-type ovarian granulosa cell tumors. Gynecol Oncol 143: 571-577. [Crossref]
- Kimura T, Shiono H, Takemoto T, Ohta Y (2013) Lung metastasis from an ovarian granulose cell tumor 36 yeas after diagnosis: report of a case. Surg today 43: 199-202. [Crossref]
- Gupta N, Rajwanshi A, Dey P, Suri V (2012) Adult granulosa cell tumor presenting as metastasis to the pleual and peritoneal cavity. Diagnostic Cytopathology 40: 912-915. [Crossref]
- Harp GM, Matwiyoff GN, Escobar SJ, Thompson KE, Alkhas A et al. (2018) An elderly woman with a mediastinal granulosa cell tumour: a rare presentation. Respiratory Case Reports 6: e00290. [Crossref]
- Lee IW, Levin W, Chapman W, Goldberg RE, Murph KJ et al. (1999) Radiotherapy for the treatment of metastatic granulosa cell tumor in the mediastinum: a case report. Gynecologic Oncology 73: 455-460. [Crossref]
- Diddle AW (1952) Granulosa- and theca-cell ovarian tumors: prognosis. Cancer 5: 215-228. [Crossref]
- Harbhajanka A, Bitterman P, Reddy VB, Park JW, Gattuso P (2016) Cytomorphology and clinicopathologic correlation of the recurrent and metastatic adult granulosa cell tumor of the ovary: A retrospective review. Diagn Cytopathol 44: 1058-1063. [Crossref]
- Young RH (2018) Ovarian Sex Cord-Stromal Tumors: Reflections on a 40-Year Experience With a Fascinating Group of Tumors, Including Comments on the Seminal Observations of Robert E. Scully, MD. Arch Pathol Lab Med 142: 1459-1484. [Crossref]
- Al-Agha OM, Huwait HF, Chow C, Yang W, Senz J et al. (2011) FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol 35: 484-494. [Crossref]
- Pujade-Laurraine E, Savina A, Maillet D, Gillet G, Treilleux I et al. (2016) DICER1 and FOXL2 mutations in ovarian sex cord-stromal tumours: a GINECO Group study. Histopathology 68: 279-285. [Crossref]