Metabolomics of Rat Brain After Treatment with Phenelzine: High-Resolution Mass Spectrometric Demonstration of Increased Brain Levels of N-Acetyl Amino Acids
A B S T R A C T
Background: Phenelzine (PLZ) is a non-specific monoamine oxidase inhibitor that has demonstrated clinical efficacy in patients with treatment resistant depression. The mechanism of action with regard to this efficacy is complicated in that its metabolite, β-phenylethylidenehydrazine (PEH), is an inhibitor of amino acid transaminases resulting in dramatic brain elevations of GABA, alanine, ornithine and tyrosine. The full neurochemical profile of PLZ and PEH remain to be explored.
Objective: To undertake a non-targeted metabolomics study of phenelzine on rat brain neurochemistry.
Methods: We undertook a high-resolution mass spectrometric metabolomics analysis of rat cortical brain 1 and 12 hours after intraperitoneal dosing with PLZ or PEH. Tandem mass spectrometry was utilized to obtain relative quantitation data.
Results: N-acetyl amino acids were found to be elevated in cortical brain tissue following either PLZ or PEH treatments.
Conclusions: Our data indicate PLZ treatment significantly augments brain levels of N-acetyl amino acids and that this may involve inhibition of deacylases by PEH and/or induction of N-amino acid acetyltransferases.
Keywords
Phenelzine, rat brain, N-acetyl amino acids
Introduction
Phenelzine is a nonspecific monoamine oxidase (MAO) inhibitor that has been utilized in patients with treatment resistant depression [1-4]. Phenelzine is a potent inhibitor of both MAO-A and MAO-B which increases brain monoamine levels. In contrast, its metabolite, β-phenylethylidenehydrazine (PEH), is a weak MAO inhibitor but is a potent transaminase inhibitor that significantly elevates brain levels of GABA and alanine, tyrosine, and ornithine [5-8]. PLZ and PEH also are both carbonyl scavenging agents that reduce reactive aldehyde toxicity in vivo and in vitro. The aldehyde scavenging actions of PLZ and PEH have been hypothesized to be responsible for their neuroprotective actions in animal models of ischemia-reperfusion injury, traumatic brain injury, spinal cord injury, experimental autoimmune encephalitis, and in vitro aldehyde toxicity [9-14]. These data indicate that PLZ and PEH have very complex pharmacodynamic effects. To further investigate the biochemical effects of these compounds, we undertook a non-targeted metabolomics study of PLZ and PEH on the rat brain metabolome. This study revealed that PLZ and PEH significantly augment brain levels of free N-acetyl amino acids.
Methods
I Rat Brain Samples
Male Sprague Dawley rats were dosed intraperitonially with 30 mg/kg of PLZ or PH, and the brains harvested after decapitation at 1 and 12 hours. The brains were frozen immediately in isopentane on solid carbon dioxide and then removed to containers stored at -80oC until the frontal cortex was dissected out for metabolomics analysis. All procedures involving animals were approved by the University of Alberta Biosciences Animal Care and Use Committee (AUP00000216) and were in accordance with the guidelines of the Canadian Council on Animal Care.
II Sample Processing
40 to 60 mg of cortical tissue were sonicated in 1 mL of ice-cold acetonitrile:methanol:formic acid (800:200:2.5) containing the stable isotope internal standards [2H3]N-acetyl-methionine, [2H5]N-acetyl-glutamate, and bromocriptine [15]. After centrifugation at 30,000 x g and 4oC for 30 min, 750 µL of the clear supernatant was dried by centrifugal vacuum evaporation. The samples were next dissolved in acetonitrile:methanol (1:1) for flow infusion analyses.
III High-Resolution Mass Spectrometric Analyses
Samples underwent flow infusion analyses at a flow rate of 12 μL per min. and were analysed via high-resolution mass spectrometry (HR-MS) utilizing a Q-Exactive benchtop orbitrap (Thermo Fisher) with a resolution of 140,000 and less than 1 ppm mass error. Negative ion electrospray ionization (NESI) with a sheath gas of 12, a spray voltage of 3.7 kV, and a capillary temperature of 321oC was used. For the pilot metabolomics analysis, the scan was from 60 to 900 amu. The data were analysed via an in-house Excel (Microsoft) spreadsheet with over 1200 metabolites of interest.
To obtain relative quantitative data of N-acetyl amino acids, MS2 studies utilized a window of 0.4 amu for the precursor ion and the product ions were acquired at high resolution (< 1 ppm mass error). For MS2 studies (Table 1) the neutral collision energy (NCE) was optimized between 20 and 30 eV.
Table 1: MS2 analyses of N-acetyl amino acids.
|
N-Acetyl Amino Acid |
[M-H]- |
MS2 Product |
Product Anion |
|
N-Acetyl Glycine |
116.0352 |
Gly |
74.0247 |
|
N-Acetyl Proline |
156.0665 |
Pro |
114.0717 |
|
N-Acetyl Valine |
158.0822 |
Val |
116.0717 |
|
N-Acetyl Threonine |
160.0615 |
Thr |
118.0509 |
|
N-Acetyl Hydroxyproline |
172.0615 |
Hydroxyproline |
130.0509 |
|
N-Acetyl Leucine |
172.0979 |
Leucine |
130.0873 |
|
N-Acetyl Glutamine |
187.0724 |
Glutamine |
145.0618 |
|
N-Acetyl Glutamate |
188.0564 |
Glutamate-CO2 |
102.0560 |
|
N-Acetyl [2H5]Glutamate |
193.0731 |
[2H5]Glutamate – CO2 |
107.0873 |
|
N-Acetyl-Methionine |
190.0547 |
Methionine |
148.0437 |
|
N-Acetyl [2H3]Methionine |
193.0731 |
[2H3]Methionine |
151.0626 |
IV Data Presentation
Data are presented as relative (R) N-acetyl amino acid levels (i.e. the ratio of the signal intensity of the endogenous N-acetyl amino acid to the signal intensity of an appropriate stable isotope internal standard), corrected for protein, ± SD (N=5).
Results
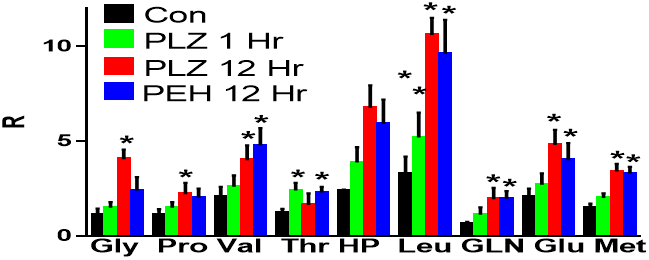
The most marked observation from our preliminary non-targeted metabolomics analysis was increased brain levels of N-acetyl amino acids with PLZ and PEH dosing. Since this was a flow infusion analysis, there were a number of potential isobars with the exact mass of each N-acetyl amino acid. Therefore, to obtain relative quantitation data we performed MS2 analyses. This approach clearly demonstrated increased levels of an array of brain N-acetyl amino acids (Figure 1). It is interesting to note that N-acetylaspartate levels, which are in millimolar (mM) concentrations in the brain, were unaffected by the drug treatments.
Figure 1: Relative levels (R) of N-acetyl amino acids in rat cortical brain tissue at 1 and 12 hours after treatment with phenelzine (PLZ, 30 mg/kg, i.p.) or β-phenylethylidenehydrazine (PEH). Gly: glycine; Pro: proline; Val: valine; Thr: threonine; Hyp: hydroxyproline; Leu: leucine/isoleucine; Gln: glutamine; Glu: glutamate; Met: methionine. Mean ± SEM (N=5); *, p < 0.05.
Discussion
While our knowledge base regarding post-translational processing of proteins, via N-acetylation of serine, alanine, glycine, methionine, threonine, valine, and aspartate (EC 2.3.1.254-258), or lysine (EC 2.3.1.32) has grown significantly, our understanding of the roles of free N-acetyl amino acids is much more limited [16, 17]. Protein bound N-acetyl amino acids are released by acylaminoacyl peptidase (EC 3.4.19.1); however, free N-acetyl amino acids are also synthesized by a number of N-acetyl transferases. The most studied free N-acetyl amino acid is N-acetylaspartate, since it is present in millimolar concentrations in the brain [18, 19]. N-Acetylaspartate is synthesized by a specific acetylase NAT8L (EC 2.3.1.17) [20, 21]. Whereas N-acetyl glutamate and N-acetylmethionine are both synthesized via amino acid N-acetyltransferase (EC 2.3.1.1) [22, 23]. Interestingly, methamphetamine, a monoamine releaser, has been shown to induce NAT8, the aforementioned synthetic enzyme involved in the biosynthesis of N-acetylaspartate [24, 25]. PLZ, which augments monoamine levels, may therefore also induce NAT8L and augment N-acetylamino acids. However, PEH which is a weak MAO inhibitor, could not be acting via this mechanism.
Alternatively, inhibition of catabolism might be involved in the augmentation of N-acetyl amino acids, similar to the augmentation of amino acids via inhibition of transaminases by PEH [7]. The potential enzyme targets include aliphatic aminoacylase (EC 3.5.1.14; ACY1) [26, 27], aspartoacylase (EC 3.5.1.15; ACY2), and N-amino aromatic amino acid amidohydrolase (EC 3.5.1.114; ACY3) [28, 29]. In this regard, inhibition of ACY3 provides neuroprotection from aldehyde toxicity, suggesting that in addition to carbonyl scavenging, augmentation of N-acetyl amino acids may contribute to the neuroprotection provided by PLZ in models of neural trauma [9-14, 29].
The functional roles of free N-acetyl amino acids remain to be more fully elucidated but decreased plasma levels of N-acetylmethionine have been monitored in cystic fibrosis patients and childhood obesity, and decreased plasma levels of N-acetylglycine in obesity [30-33]. Cadmium, which is an ACY1 inhibitor, elevates urinary levels of N-acetylglutamate, N-acetylglutamine, and N-acetylphenylalanine, suggesting a rapid turnover rate for these N-acetyl amino acids in vivo [34, 35]. Similarly, precursor labeling studies have defined the rapid dynamics of N-acetylmethionine synthesis in human oligodendrocyte cultures [22].
A detailed analysis of N-acetyl amino acids in dogs with gallbladder mucocele formation found decreased blood levels of N-acetylated alanine, glycine, glutamate, isoleucine, leucine, methionine, serine, and threonine [35]. In contrast, the bile in these dogs was characterized by increased levels of N-acetylated glutamate, histidine, isoleucine, leucine, lysine, threonine, tryptophan, tyrosine, and valine. These data suggest that N-acetyl amino acids play a complex metabolic function in the gallbladder.
With regard to brain function, free N-acetylmethionine, N-acetylglutamine, N-acetylglutamate, N-acetylasparagine, and N-acetylalanine have all been monitored in the human brain [23, 36-40]. N-acetylglutamate is a critical modulator of the urea cycle, while N-acetylleucine modulates the neuronal activity of vestibulocerebellar and posterolateral thalamic circuits involved in vestibular function, while N-acetylglutamine appears to be involved in the sleep-wake cycle [40-42]. Clearly, we currently have limited knowledge of the roles of N-acetyl amino acids in brain function.
Conflicts of Interest
None.
Funding
This research was funded by Lincoln Memorial University, the University of Alberta, and a grant to GBB from the Canadian Institutes of Health Research (CIHR; grant MOP-86712).
Article Info
Article Type
Research ArticlePublication history
Received: Sat 20, Jun 2020Accepted: Tue 07, Jul 2020
Published: Fri 24, Jul 2020
Copyright
© 2023 Paul L. Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.NNB.2020.03.03
Author Info
Paul L. Wood John E. Cebak Glen B. Baker
Corresponding Author
Paul L. WoodMetabolomics Unit, College of Veterinary Medicine, Lincoln Memorial University, Tennessee, USA
Figures & Tables
Table 1: MS2 analyses of N-acetyl amino acids.
|
N-Acetyl Amino Acid |
[M-H]- |
MS2 Product |
Product Anion |
|
N-Acetyl Glycine |
116.0352 |
Gly |
74.0247 |
|
N-Acetyl Proline |
156.0665 |
Pro |
114.0717 |
|
N-Acetyl Valine |
158.0822 |
Val |
116.0717 |
|
N-Acetyl Threonine |
160.0615 |
Thr |
118.0509 |
|
N-Acetyl Hydroxyproline |
172.0615 |
Hydroxyproline |
130.0509 |
|
N-Acetyl Leucine |
172.0979 |
Leucine |
130.0873 |
|
N-Acetyl Glutamine |
187.0724 |
Glutamine |
145.0618 |
|
N-Acetyl Glutamate |
188.0564 |
Glutamate-CO2 |
102.0560 |
|
N-Acetyl [2H5]Glutamate |
193.0731 |
[2H5]Glutamate – CO2 |
107.0873 |
|
N-Acetyl-Methionine |
190.0547 |
Methionine |
148.0437 |
|
N-Acetyl [2H3]Methionine |
193.0731 |
[2H3]Methionine |
151.0626 |
References
- Jonathan M Meyer, Michael A Cummings, George Proctor (2017) Augmentation of phenelzine with aripiprazole and quetiapine in a treatment-resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr 22: 391-396. [Crossref]
- Shulman KI, Herrmann N, Walker SE (2013) Irreversible monoamine oxidase inhibitor (MAOI) antidepressants have significant efficacy in treatment-resistant unipolar depression, Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27: 789-797.
- Samantha J Thomas, Mirae Shin, Melvin G McInnis, Jolene R Bostwick (2015) Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy 35: 433-449. [Crossref]
- David Menkes, Peter Bosanac, David Castle (2016) MAOIs - does the evidence warrant their resurrection? Australas Psychiatry 24: 371-373. [Crossref]
- Kathryn G Todd, Glen B Baker (2008) Neurochemical effects of the monoamine oxidase inhibitor phenelzine on brain GABA and alanine: A comparison with vigabatrin. J Pharm Pharm Sci 11: 14s-21s. [Crossref]
- Dmitriy Matveychuk, Emerson Nunes, Nasir Ullah, Carlos A Velázquez-Martinez, Erin M MacKenzie et al. (2013) Comparison of phenelzine and geometric isomers of its active metabolite, β-phenylethylidenehydrazine, on rat brain levels of amino acids, biogenic amine neurotransmitters and methylamine. J Neural Transm 120: 987-996. [Crossref]
- Dmitriy Matveychuk, Emerson Nunes, Nasir Ullah, Fahad S Aldawsari, Carlos A Velázquez Martínez et al. (2014) Elevation of rat brain tyrosine levels by phenelzine is mediated by its active metabolite β-phenylethylidenehydrazine. Prog Neuropsychopharmacol Biol Psychiatry 53: 67-73. [Crossref]
- Erin M MacKenzie, Suzanne L Grant, Glen B Baker, Paul L Wood (2008) Phenelzine causes an increase in brain ornithine that is prevented by prior monoamine oxidase inhibition. Neurochem Res 33: 430-436. [Crossref]
- Paul L Wood, M Amin Khan, Joseph R Moskal, Kathryn G Todd, Véronique A M I Tanay et al. (2006) Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res 1122: 184-190. [Crossref]
- Indrapal N Singh, Lesley K Gilmer, Darren M Miller, John E Cebak, Juan A Wang et al. (2013) Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J Cereb Blood Flow Metab 33: 593-599. [Crossref]
- Zhe Chen, Jonghyuck Park, Breanne Butler, Glen Acosta, Sasha Vega Alvarez et al. (2016) Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J Neurochem 138: 328-338. [Crossref]
- Curtis A Benson, Grace Wong, Gustavo Tenorio, Glen B Baker, Bradley J Kerr (2013) The MAO inhibitor phenelzine can improve functional outcomes in mice with established clinical signs in experimental autoimmune encephalomyelitis (EAE). Behav Brain Res 252: 302-311. [Crossref]
- Mee-Sook Song, Glen B Baker, Serdar M Dursun, Kathryn G Todd (2010) The antidepressant phenelzine protects neurons and astrocytes against formaldehyde-induced toxicity. J Neurochem 114: 1405-1413. [Crossref]
- Glen Baker, Dmitriy Matveychuk, Erin M MacKenzie, Andrew Holt, Yanlin Wang (2019) Attenuation of the effects of oxidative stress by the MAO-inhibiting antidepressant and carbonyl scavenger phenelzine. Chem Biol Interact 304: 139-147. [Crossref]
- Drazic A, Myklebust LM, Ree R, Arnesen T. (2016) The world of protein acetylation. Biochim Biophys Acta 1864: 1372-1401. [Crossref]
- Petra Van Damme, Marta Lasa, Bogdan Polevoda, Cristina Gazquez, Alberto Elosegui-Artola et al. (2012) N-terminal acetylome analyses and functional insights of the N-terminal acetyltransferase NatB. Proc Natl Acad Sci U S A 109: 12449-12454. [Crossref]
- Linda Chang, Joseph Friedman, Thomas Ernst, Kai Zhong, Nicholas D Tsopelas et al. (2007) Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry 62: 1396-1404. [Crossref]
- Lucia Margari, Andrea De Giacomo, Francesco Craig, Roberto Palumbi, Antonia Peschechera et al. (2018) Frontal lobe metabolic alterations in autism spectrum disorder: a 1 H-magnetic resonance spectroscopy study. Neuropsychiatr Dis Treat 14: 1871-1876. [Crossref]
- Ariane R Pessentheiner, Helmut J Pelzmann, Evelyn Walenta, Martina Schweiger, Lukas N Groschner et al. (2013) NAT8L (N-acetyltransferase 8-like) accelerates lipid turnover and increases energy expenditure in brown adipocytes. J Biol Chem 288: 36040-36051. [Crossref]
- Yoshiaki Miyamoto, Noriyuki Iegaki, Kequan Fu, Yudai Ishikawa, Kazuyuki Sumi et al. (2017) Striatal N-acetylaspartate synthetase Shati/Nat8l regulates depression-like behaviors via mGluR3-mediated serotonergic suppression in mice. Int J Neuropsychopharmacol 20: 1027-1035. [Crossref]
- Ljubica Caldovic, Nicholas Ah Mew, Dashuang Shi, Hiroki Morizono, Marc Yudkoff et al. (2010) N-acetylglutamate synthase: structure, function and defects. Mol Genet Metab 100: S13-S19. [Crossref]
- Tara Smith, M Said Ghandour, Paul L Wood (2011) Detection of N-acetyl methionine in human and murine brain and neuronal and glial derived cell lines. J Neurochem 118: 187-194. [Crossref]
- Prasanth S Ariyannur, John R Moffett, Pachiappan Manickam, Nagarajan Pattabiraman, Peethambaran Arun et al. (2010) Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res 1335: 1-13. [Crossref]
- Kyosuke Uno, Toh Miyazaki, Kengo Sodeyama, Yoshiaki Miyamoto, Atsumi Nitta (2017) Methamphetamine induces Shati/Nat8L expression in the mouse nucleus accumbens via CREB- and dopamine D1 receptor-dependent mechanism. PLoS One 12: e0174196. [Crossref]
- H Lindner, S Höpfner, M Täfler Naumann, M Miko, L Konrad et al. (2000) The distribution of aminoacylase I among mammalian species and localization of the enzyme in porcine kidney. Biochimie 82: 129-137. [Crossref]
- Jörn Oliver Sass, Jathana Vaithilingam, Corinne Gemperle Britschgi, Cathérine C S Delnooz, Leo A J Kluijtmans et al. (2016) Expanding the phenotype in aminoacylase 1 (ACY1) deficiency: characterization of the molecular defect in a 63-year-old woman with generalized dystonia. Metab Brain Dis 31: 587-592. [Crossref]
- Jeremy S Francis, Ana Olariu, Scott W McPhee, Paola Leone (2006) Novel role for aspartoacylase in regulation of BDNF and timing of postnatal oligodendrogenesis. J Neurosci Res 84: 151-169. [Crossref]
- Kirill Tsirulnikov, Natalia Abuladze, Anatol Bragin, Kym Faull, Duilio Cascio et al. (2012) Inhibition of aminoacylase 3 protects rat brain cortex neuronal cells from the toxicity of 4-hydroxy-2-nonenal mercapturate and 4-hydroxy-2-nonenal. Toxicol Appl Pharmacol 263: 303-314. [Crossref]
- Theresa A Laguna, Cavan S Reilly, Cynthia B Williams, Cole Welchlin, Chris H Wendt (2015) Metabolomics analysis identifies novel plasma biomarkers of cystic fibrosis pulmonary exacerbation. Pediatr Pulmonol 50: 869-877. [Crossref]
- E Isganaitis, S L Rifas Shiman, E Oken, J M Dreyfuss, W Gall et al. (2015) Associations of cord blood metabolites with early childhood obesity risk. Int J Obes 39: 1041-1048. [Crossref]
- Steven C Moore, Charles E Matthews, Joshua N Sampson, Rachael Z Stolzenberg Solomon, Wei Zheng (2014) Human metabolic correlates of body mass index. Metabolomics 10: 259-269. [Crossref]
- Nancy F Butte, Yan Liu, Issa F Zakeri, Robert P Mohney, Nitesh Mehta et al. (2015) Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr 102: 256-267. [Crossref]
- Peter Curley, Chris van der Does, Arnold J M Driessen, Jan Kok, D van Sinderen (2003) Purification and characterisation of a lactococcal aminoacylase. Arch Microbiol 179: 402-408. [Crossref]
- Sailendra Nath Sarma, Ammar Saleem, Jin Yong Lee, Maki Tokumoto, Gi Wook Hwang et al. (2018) Effects of long-term cadmium exposure on urinary metabolite profiles in mice. J Toxicol Sci 43: 89-100. [Crossref]
- Jody L Gookin, Kyle G Mathews, John Cullen, Gabriela Seiler (2018) Qualitative metabolomics profiling of serum and bile from dogs with gallbladder mucocele formation. PLoS One 13: e0191076. [Crossref]
- J V Auditore, L Wade (1973) Isolation of N-acetylglutamine, pyroglutamic acid and the butyl ester of pyroglutamic acid from human brain. J Neurochem 21: 335-343. [Crossref]
- J V Auditore, L Wade, E J Olson (1966) Occurrence of N-acetyl-L-glutamic acid in the human brain. J Neurochem 13: 1149-1155. [Crossref]
- J V Auditore, L H Wade (1972) N-acetyl-L-asparagine in human brain. Neuropharmacology 11: 385-394. [Crossref]
- J V Auditore, L Wade (1971) Occurrence of N-acetylalanine in the human brain. J Neurochem 18: 2389-2395. [Crossref]
- Lisa Günther, Roswitha Beck, Guoming Xiong, Heidrun Potschka, Klaus Jahn et al. (2015) N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS One 10: e0120891. [Crossref]
- Allen K Bourdon, Giovanna Maria Spano, William Marshall, Michele Bellesi, Giulio Tononi et al. (2018) Metabolomic analysis of mouse prefrontal cortex reveals upregulated analytes during wakefulness compared to sleep. Sci Rep 8: 11225. [Crossref]
- Wood PL (2020) Flow injection ESI high-resolution mass spectrometry metabolomics analytical platform. IN Springer Protocols, Neuromethods: Metabolomics in press (PL Wood, Editor).
