Menopausal Status Impairs Acute Inflammatory Recovery from Resistance Exercise
A B S T R A C T
The purpose of this study was to explore the effects of menopausal status on inflammatory responses to a single bout of resistance exercise (RE). Twenty pre- (N=10) or post- menopausal (N=10) women aged 18-65 years had one repetition maximum (1RM) determined for the chest and leg press, leg and biceps curl, vertical pull down, triceps and leg extension exercises. On a separate day, participants completed a session of full body resistance exercise (RE) during which subjects performed three sets of 10 repetitions at 75% 1RM for the aforementioned exercises. Blood samples were obtained prior to, immediately, and one hour after exercise. Changes in interleukin-6, -10, and transforming growth factor beta (IL-6, -10, and TGF-Β1) were determined via enzyme-linked immunosorbent assays (ELISA). IL-6 significantly increased (P<0.05) over time in both groups. Immediately post exercise levels of IL-10 were significantly lower (P<0.05) in the post-menopausal group. Changes in IL-10 correlated with 17Β-estradiol levels (r2=0.45, P<0.001). Menopausal status impaired inflammatory recovery following acute RE. These changes may be attributed to menopause-induced perturbations to the hormonal milieu.
Keywords
Cytokines, inflammation, menopause, resistance exercise
Introduction
Menopause encapsulates the life-altering process whereby loss of ovarian follicles facilitates a number of permanent biochemical and physiologic changes. Though a highly individual process, clinically, menopause can be identified by the cessation of menses in conjunction with systemic changes in inhibin B, anti-mÜllerian hormone (AMH), follicle stimulating hormone (FSH) and estrogens. Over two million women in the United States transition into menopause yearly, equating to roughly 6,000 individuals daily. With the vast increase in life expectancy over the last century, women spend nearly 40% of their lives in the post-menopausal state.
Though a natural process, menopause is associated with an elevated risk of osteoporosis, cardiovascular disease, gastrointestinal distress, chronic low-grade inflammation and sarcopenia [1]. The risk of disease onset increases in the presence of other pre-existing chronic conditions, primarily insulin resistance and obesity [2]. The precise mechanisms underlying menopause-induced disease risk remains unclear. However, evidence thus points toward the role of estrogen deficiency and inflammation in the post-menopausal state [3].
Several forms of estrogens exist, 17Β-estradiol (E2) being the most biologically active and relevant. Transition into menopause causes variable fluctuations in circulating E2, until finally concentrations drop and remain low in otherwise healthy individuals. Other than development and maintenance of reproductive tissues, E2 assumes a number of biological roles including skeletomuscular remodeling, lipid metabolism, immune function, and inflammatory regulation [4].
Cytokines are a group of small, ubiquitously expressed and secreted pro- and anti-inflammatory proteins responsible for orchestrating chemical signal transduction [5]. In response to acute, exercise-induced muscle contraction, cytokines are secreted from skeletal muscle into the bloodstream, promoting numerous cellular and physiologic adaptations [6]. This action continues until anti-inflammatory proteins are recruited to the injury site to resolve the acute phase response and promote inflammatory recovery [7]. Under certain chronic physiological stressors, such as metabolic and/or cardio-pulmonary disorders, the body may consistently overproduce specific cytokines, a condition referred to as chronic, low-grade inflammation [8].
Inflammation serves as a target for risk reduction treatment due to the association with menopause-induced disease [9]. Resistance exercise (RE) is the primary lifestyle means of maintaining and improving musculoskeletal mass [10]. Also, RE promotes a number of other positive physiologic adaptations, including improvements in strength, neuromuscular coordination, proprioception, body composition, metabolic rate, immune function and inflammatory control [11]. Interestingly, these changes have been observed independently of changes in body mass [12]. Thus, RE programs may present a unique opportunity to reduce risk of disease progression, while providing numerous other health benefits. Exercise also provides a stress model by which biological mechanisms may be further tested and illuminated.
Thus, the purpose of this study was to explore the effects of menopausal status on inflammatory responses to a single bout of RE. It was hypothesized that post-menopausal women will differ in their cytokine response to acute RE when compared to pre-menopausal women. It was also hypothesized that post-menopausal women will have increased baseline pro-inflammatory plasma cytokine concentrations in comparison to pre-menopausal women.
Materials and Methods
I Subjects
Twenty women, 10 pre-menopausal and 10 post-menopausal, were selected based upon the following exclusionary criteria: history of cardiovascular or pulmonary disease, stroke, autoimmune disorders, chronic infection, smokers or smokeless tobacco users, surgery within 5 years, use of anti-inflammatory medications, hormone replacement, hormonal birth control in the past 6 months, inflammatory disease, pre, type 1 or type 2 diabetes, and a body fat >35%.
Participants had not engaged in any structured resistance and/or aerobic training programs for at least six months prior to beginning the study. Structured exercise was defined as two or more days per week of moderate intensity resistance (>70% one repetition maximum, 1RM) or aerobic (>60% maximal oxygen consumption, VO2MAX) exercise. Enrolled participants were not currently engaged in any high intensity training. Participants reported varying levels of recreational activity, including but not limited to, yoga, Pilates, hiking, swimming, jogging, and walking. The participants were free from musculoskeletal injury.
Participants were recruited using flyers and regular notifications during lectures at local universities. All participants received and signed an informed consent form that was approved by the Institutional Review Board (IRB), and were free to leave the study at any time.
II Menopausal Status
Participants were categorized as either pre- or post-menopausal based upon history of menopausal status and plasma follicle stimulating hormone (FSH) levels. Pre-menopausal was defined as having a regular, monthly menstrual cycle during the past six months and a FSH level of less than 25 mIU/ml. Post-menopausal was defined as not having a menstrual cycle for the past three years, as a result of natural menopause, and a FSH level of 25 mIU/ml or greater [13].
III Body Composition
Body composition was determined using whole body air-displacement plethysmography with the BOD POD® system (COSMED USA Inc.; Concord, California). On the day of testing, subjects were instructed to arrive at the Human Performance Laboratory between 7 and 9 am after a 10-hour overnight fast. The BOD POD and weight scale were calibrated prior to each individual test. All subjects wore minimal, form fitting clothing and a swim cap during testing according to device guidelines. During measurement, subjects were instructed to sit still with their hands resting upon their thighs and breathe normally. Whole body density was used to determine percent body fat using the Siri equation:
% Body Fat = (495 / Body Density) - 450.
Waist to hip ratio (WHR) was determined by measuring the circumference of the narrowest part of the waist and dividing this by the measurement of the largest circumference of the hips. A Gulick measuring tape was utilized for all circumference measurements.
IV Metabolic Panel
Total cholesterol (TC), high density lipoprotein (HDL) cholesterol, triglycerides (TRG), TC/HDL ratio, non-HDL cholesterol, estimated LDL cholesterol and blood glucose were determined via the Cholestech LDX Lipid Profile GLU Test (Alere, Waltham, MA). Blood samples were obtained from a fingerstick and processed according to the manufacturer's instructions. Samples were analyzed using the Cholestech LDX® System (Alere, Waltham, MA).
V Blood Sampling
Subjects reported to the Human Performance Laboratory in the morning after an overnight fast for the baseline blood sample. Blood samples were obtained from the antecubital vein immediately prior to (PRE), immediately after (POST), and one hour (1 HOUR POST) after the termination of exercise. Samples were collected in 6 mL BD Vacutainer® tubes treated with K2 EDTA. Whole blood was then centrifuged for 10 minutes at 1000 x g in 4°C. The plasma was drawn off in 500 μL aliquots and then immediately transferred and stored in a -80°C freezer until the time of analysis.
VI Blood Analysis
For analysis, samples were thawed and centrifuged for 30 seconds at 1000 x g at 4°C for purification and separation. Samples underwent no more than two freeze thaw cycles during the span of the analysis. Plasma concentrations of IL-6, IL-10, TGF-Β1, E2, and FSH were then assessed using commercially available enzyme-linked immunosorbent assays (ELISA) (IL-6 & TGF-Β1, R&D Systems®, Minneapolis, MN; IL-10, eBioscience®, San Diego, CA; E2, Thermo Fisher Scientific, Waltham, MA; FSH, Ray Biotech, Norcross, GA). The sensitivities of the assays were 0.11, 0.17, 15.4, 5.0, and 8.0 pg/mL for IL-6, IL-10, TGF-Β1, E2, and FSH, respectively. The intra assay coefficients of variation (CV) for each kit were 5.8%, 8.8%, 3.0%, 5.3%, and 5.2% for IL-6, IL-10, TGF-Β1, E2, and FSH, respectively. High sensitivity kits were used when available and appropriate.
VII Strength Assessment
On a separate day, at least two or more weeks prior to the training bout, participants came in to the Human Performance Laboratory for exercise acclimation and completed a 1RM test battery which consisted of the following exercises: bilateral leg press, seated chest press, knee extension, knee flexion, triceps extension, biceps curl and vertical pull down exercise. All exercises were performed on Icarian cable-based weight stack machines (Precor, Woodinville, WA). After a dynamic warm up, subjects began with a submaximal load and progressed until failure. One minute of rest was allotted between each attempt. The last completed load prior to failure was considered the subject's 1RM. The exercise sequence was the same for each participant.
VIII Resistance Exercise Protocol
On the day of testing, participants arrived after an overnight fast and sat quietly for 5 minutes. After the initial blood draw, subjects performed 5 minutes of sub-maximal walking. The walking speed was selected by the subject and ranged from two to three miles per hour (mph). After a brief stretching and dynamic warm up period, the subjects performed three sets of 10 repetitions of the supine chest press, bilateral leg press, seated knee extension, prone knee flexion, seated triceps extension, biceps curl and vertical pull down exercise machines. Exercise intensity was set at 75% 1RM. If the subject was not able to complete all of the prescribed repetitions, the load was reduced to the next available weight and the subject continued until completion of 10 repetitions. Participants followed a 2/0/2 tempo pattern with approximately 60-second rest intervals between sets. Pre-menopausal subjects were tested on the 7th day (mid follicular phase; ± 1 day) of their menstrual cycle.
IX Statistical Analysis
An independent t-test was used to determine baseline differences between groups. A mixed design, two-way repeated measures analysis of variance (ANOVA) was used to assess differences between groups in cytokine concentrations across three time periods (pre, post, and 1 hour post). The Wilks-Shapiro test was used to assess normality of the sample population. Mauchly's sphericity test was used to assess homogeneity of variance between groups. The Greenhouse-Geisser correction was used in cases where sphericity was violated. When significant main effects for time or time by group interactions were revealed, post-hoc analysis was conducted using the Bonferroni procedure. Associations between variables were determined using Pearson product-moment correlation coefficient. Associations for non-normal distributions were assessed using Spearman's rank-order correlation coefficient. Correlations are displayed as individual plots with linear regression and 95% confidence bands. Tables and figures are displayed as the mean ± standard error. GraphPad Prism (version 5.0) was used for all analyses with .05 used as the level of significance.
Results
Subject characteristics are displayed in (Table 1). Results for IL-6, IL-10, and TGF-Β are presented in (Figures 1, 2, & 3) respectively. Correlations are displayed in (Figure 4).
Table 1: Subject Characteristics.
|
Characteristic |
Pre-Menopausal |
Post-Menopausal |
p-value (2-tailed) |
||
|
M |
SEM |
M |
SEM |
||
|
Age (yrs) |
27 |
1.0 |
59 |
0.8 |
<0.0001* |
|
Height (cm) |
164.4 |
2.3 |
164.1 |
1.7 |
0.90 |
|
Body Mass (kg) |
60.5 |
2.9 |
60.1 |
2.7 |
0.93 |
|
Fat Free Mass (kg) |
45.9 |
1.7 |
43.1 |
1.5 |
0.24 |
|
Fat % |
23.7 |
1.5 |
28.3 |
1.9 |
0.04* |
|
Waist to Hip Ratio |
0.73 |
0.0 |
0.80 |
0.0 |
0.005* |
|
Blood Glucose (mg/dL) |
83.3 |
2.0 |
89.5 |
2.5 |
0.13 |
|
Total Cholesterol (mg/dL) |
167.7 |
9.6 |
183.5 |
7.6 |
0.22 |
|
LDL-C (mg/dL) |
100.8 |
9.5 |
97.0 |
5.6 |
0.73 |
|
HDL-C (mg/dL) |
59.8 |
7.0 |
66.8 |
4.4 |
0.41 |
|
Triglycerides (mg/dL) |
65.7 |
10.5 |
80.6 |
13.3 |
0.39 |
|
FFM-Adjusted 1RM Index |
7.89 |
0.5 |
6.53 |
0.3 |
0.026* |
|
FSH (mIU/mL) |
5.2 |
0.9 |
58.0 |
8.0 |
<0.0001* |
|
17β-estradiol (pg/mL) |
109.8 |
13.5 |
17.7 |
4.9 |
0.001* |
|
Data are presented as mean ± standard error. LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, FFM: fat free mass, 1RM: one repetition maximum, FSH: follicle stimulating hormone. FFM-Adjusted 1RM index was calculated by dividing an individual’s raw total (kg) by their fat free mass (kg). *P<0.05 between groups. |
|||||
There were significant differences in age (p<0.0001), waist to hip ratio (P<0.01), body fat (P<0.05), FFM-adjusted 1RM index (P<0.05), FSH (P<0.0001), and 17Β-estradiol (P<0.001) (Table 1). The post-menopausal group was significantly older than the pre-menopausal group (range: pre-menopausal = 23-32, post-menopausal = 56-65 years), as well as exhibiting a significantly greater waist to hip ratio, body fat, FSH, and lower 17Β-estradiol. The pre-menopausal group was significantly stronger than the post-menopausal group both in absolute and FFM-adjusted values (range: pre-menopausal = 6.21-11.65, post-menopausal = 5.20-7.89 arbitrary units). There were no significant baseline differences in IL-6, IL-10, or TGF-Β between groups.
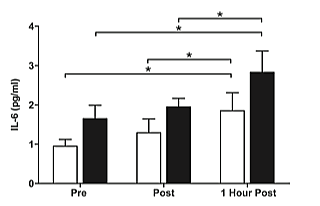
Figure 1: Plasma concentrations of IL-6 prior to, immediately after, and one hour after the cessation of resistance exercise in pre- ( white) and postmenopausal (∎black) women. *(P<0.05) indicates significant effect of time.
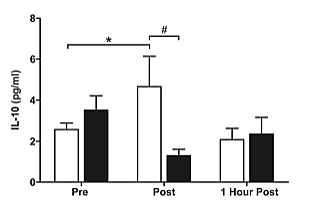
There was a significant main effect for time (P<0.05), with both groups exhibiting increased plasma concentrations of IL-6 pre to post exercise (Figure 1). Post hoc analysis revealed a significant increase (P<0.05) in IL-6 pre to 1 hour post exercise, as well as immediately post to 1 hour post exercise in both groups. There was no significant group by time interaction. There was a significant main effect for time (P< 0.05) with the pre-menopausal group displaying increased plasma concentrations of IL-10 pre to post exercise followed by a reduction to near basal levels 1 hour post exercise (Figure 2). Post hoc analysis revealed a significant increase (P<0.05) in IL-10 pre to immediately post exercise. A significant, main interaction effect (P<0.05) was also observed. Post hoc analysis further identified a significant interaction for IL-10 immediately post exercise between groups, such that the pre-menopausal group significantly increased after exercise, while the post-menopausal group decreased but not significantly.
Figure 2: Plasma concentrations of IL-10 prior to, immediately after, and one hour after the cessation of resistance exercise in pre- ( white) and postmenopausal (∎black) women. *(P<0.05) indicates significant effect of time. #(P<0.05) indicates significant time by group interaction.
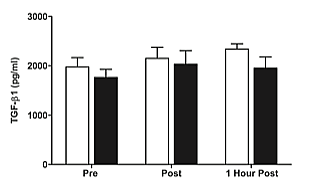
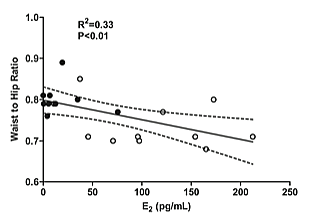
TGF-Β1 levels increased slightly, but not significantly, pre to post exercise in both groups. No significant effects for time or time by group interactions were observed for TGF-Β1 (Figure 3). Bivariate analysis of study variables revealed a moderate, significant positive correlation (r2=0.33, P=<0.01) between E2 and WHR, as well as E2 and change in IL-10 (r2=0.45, P<0.001) from pre to immediately post exercise (Figure 4).
Figure 3: Plasma concentrations of TGF-Β1 prior to, immediately after, and one hour after the cessation of resistance exercise in pre- ( white) and postmenopausal (∎black) women.
Figure 4: A) Significant correlation (P<0.01, r2=0.33) between E2 and waist to hip ratio in pre- (◦white) and post-menopausal ( •black) women. B) Significant correlation (P<0.001, r2=0.687) between E2 and change in IL-10 concentration from pre to immediately post exercise in pre- (◦white) and postmenopausal ( •black) women.
Discussion
Menopause is characterized by a loss of E2 synthesis from reproductive tissues. Given the role of E2 in tissue repair, menopause associated reductions in E2 may result in loss of inflammatory recovery in post-menopausal women. We therefore examined if menopausal status influenced the inflammatory response to acute RE-induced stress. This study specifically examined changes in plasma concentrations of IL-6, IL-10, and TGFΒ1 over time. It was hypothesized that post-menopausal women would display altered inflammatory recovery, characterized by either elevated IL-6 or decreased IL-10. It was also hypothesized that post-menopausal women would display a basal low-grade inflammation phenotype. Overall, we observed differences in the inflammatory response between pre- and post-menopausal women, supporting our first hypothesis. However, contrary to our second hypothesis, there were no baseline differences.
I IL-6 Response to RE
Significant elevations in IL-6 concentrations over time were observed in both pre and post-menopausal women. Skeletal muscle secretes IL-6 in response to exercise-induced stress and accounts for the majority of the circulatory protein. Under basal conditions, skeletal muscle IL-6 mRNA is hardly detectable. Upon onset of skeletal muscle contraction, IL-6 mRNA levels increase exponentially up to 12 hours post exercise [14]. Plasma IL-6 has been widely demonstrated to increase significantly upon an acute bout of aerobic exercise (AE) [15]. However, variable results have been reported for RE. Initial findings suggested no acute RE induced changes in IL-6 concentrations occurred in post-menopausal women [16]. However, several other studies described significant changes following RE, with an increase into the recovery period in both post-menopausal women and the general population [12, 17, 18]. The latter studies employed hypertrophy-oriented, high intensity RE protocols and revealed changes as described herein. It appears that factors such as repetition range, rest interval, contraction tempo and relative intensity may influence the magnitude of IL-6 induction.
II IL-10 Response to RE
Interleukin-10 is an anti-inflammatory cytokine that suppresses the induction of several pro-inflammatory cytokines [19]. Herein we reported that pre-menopausal women displayed significantly elevated plasma IL-10 concentrations immediately post exercise. Prolonged AE induces a marked increase in IL-10 concentration [20, 21]. However, multiple acute exercise studies have reported no significant changes in IL-10 activity [22-24]. In contrast, several other groups have reported significant changes after RE [25-27]. Hirose et al. (2014) reported significant increases in IL-10 one hour after performing moderate intensity RE. Izquierdo et al. (2009) found significantly increased post-exercise levels of IL-10 after a seven week RE intervention. Jajtner et al. (2015) found elevated plasma concentrations of IL-10 30 minutes after high volume lower body RE. Interestingly, amongst the studies reviewed there was a trend in elevated post-exercise IL-10 in trained individuals, providing a potential mechanism for training induced improvements to post-exercise inflammatory recovery and immunity.
The majority of studies assessing IL-10 responses to acute RE included males only. For the limited investigations including women, experimental control of key confounders such as regularity of menstrual cycle, day of testing in relation to menstrual phase, use of hormonal birth control, history of RE, and fasting status appeared lacking. Overall, variable exercise protocols and lack of data with well-controlled cohorts limits generalizability and warrants further experimentation.
III Menopausal Mechanisms for Exercise-Induced IL-10 Variation
Significant differences in IL-10 concentrations immediately post-exercise were observed. Stress-induced impairments in post-menopausal IL-10 production may be attributed to altered transcription or post-transcriptional modifications in immune cells regulated by E2. Immune cells rapidly infiltrate skeletal muscle, connective tissue, and bone after RE [28]. Adequate response by immune cells ensures proper tissue healing and recovery, as well as musculoskeletal adaptation to exercise. E2 is ubiquitously expressed in immune cells [29]. Upon activation, E2 binds to its alpha, beta, or plasma membrane receptor (ERa, ERΒ, or plasma membrane ER), leading to homo- or hetero-dimerization and subsequent phosphorylation. The E2 complex then translocates to the nucleus and regulates transcription. The specific complex formation of E2 is dependent upon receptor activation, binding domain and activity, and ligand involvement. E2 action is further augmented by a number of receptor mediated post-transcriptional modifications.
E2 is a known regulator of IL-10 transcription in neutrophils, macrophages, dendritic, and type 2 T helper (Th2) cells [30]. Whole blood and isolated dendrites exposed to inflammatory stimuli exhibited enhanced IL-10 secretion in the presence of E2 in a dose-dependent manner [31-33]. Skeletal muscle tissue from Sprague-Dawley rats displayed reduced neutrophil infiltration after exercise when treated with E2 [34]. More recently, macrophages treated with E2 exhibited enhanced immune recovery and were protected against endotoxins [35]. Cell and animal models in conjunction with our current data support the hypothesis that E2 may be an essential regulator of inflammatory recovery, potentially through an IL-10 mediated pathway. Further research is needed to determine the direct or indirect mechanisms of E2 inflammatory regulation.
IV Menopause Induced Visceral Adiposity
Secondary analysis of study data revealed a significantly greater body fat % and WHR in post-menopausal women, despite similarities in total body mass. WHR also negatively correlated with E2. Prior to menopause, females display greater subcutaneous & lower visceral fat accumulation than males [36]. The onset of menopause leads to a distinct phenotypic shift characterized by increased visceral adiposity. It is well established that estrogen deficiency from reproductive sites augments synthesis in peripheral tissues [37]. Extragonadal sites, such as adipose tissue, synthesize E2 via aromatization of androgens and activity of the enzyme aromatase [38, 39]. Aromatic activity in adipose tissue is regulated by a number of factors, including local cytokine production, promoter activity, and lipid mediators such as prostaglandin E2 (PGE2) [40]. Both IL-6 and TNF-a secretion from adipose tissue have been implicated in the activity of aromatic enzymes [41, 42]. Though no circulatory differences in IL-6 were observed, it remains possible that IL-6 from adipose depots is altered after menopause, driving visceral adiposity as a result of excess fat accumulation from reproductive estrogen deficiency.
In conclusion, an acute bout of moderate intensity total body RE resulted in significant changes to the cytokine milieu. Menopause did not alter IL-6 production in response to exercise-induced stress. Pre-menopausal women exhibited enhanced inflammatory resolution immediately post-exercise illustrated by increased IL-10 production. Though mechanistically unclear, recent developments in cytokine biology have elucidated a potential estrogen-dependent signaling pathway ultimately resulting in stress-induced IL-10 production. Thus, enhancing IL-10 secretion, via RE training and/or pharmacologic intervention, may provide clinical therapeutic value in estrogen deficient, post-menopausal women.
Acknowledgements
We thank the study volunteers and Cleveland State University Human Performance Laboratory staff for their time and dedication to the project. C.L. Axelrod, K.E. Sparks, K.D. Little, and E.L. Kullman recruited subjects and completed exercise testing. C.L. Axelrod and E.L. Kullman designed the study, performed data collection, analysis, and interpretation. E.L. Kullman supervised data collection, analysis, and interpretation. C.L. Axelrod drafted the manuscript and all authors contributed to editing and finalization of the manuscript. E.L. Kullman is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Christopher L. Axelrod is now affiliated with the Pennington Biomedical Research Center, Department of Translational Services, 6400 Perkins Road, Baton Rouge, LA, 70808.
Abbreviation
1RM: 1 repetition maximum
AE: aerobic exercise
AMH: anti-mÜllerian hormone
ANOVA: analysis of variance
E2: 17Β-estradiol
ELISA: enzyme-linked immunosorbent assay
FFM: fat free mass
FSH: follicle stimulating hormone
HDL: high density lipoprotein cholesterol
IL-6: interleukin-6
IL-10: interleukin-10
IRB: institutional review board
LDL: low density lipoprotein cholesterol
RE: resistance exercise
TC: total cholesterol
TGF- Β1: transforming growth factor beta-1
TRG: triglycerides
VO2max: maximal oxygen consumption
WHR: waist to hip ratio
Article Info
Article Type
Research ArticlePublication history
Received: Wed 11, Mar 2020Accepted: Sat 21, Mar 2020
Published: Mon 30, Mar 2020
Copyright
© 2023 Emily Kullman. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.GGR.2020.01.04
Author Info
Christopher L. Axelrod Emily Kullman Kathleen D. Little Kenneth E. Sparks
Corresponding Author
Emily KullmanDepartment of Health and Human Performance, Cleveland State University, Cleveland, Ohio, USA
Figures & Tables
Table 1: Subject Characteristics.
|
Characteristic |
Pre-Menopausal |
Post-Menopausal |
p-value (2-tailed) |
||
|
M |
SEM |
M |
SEM |
||
|
Age (yrs) |
27 |
1.0 |
59 |
0.8 |
<0.0001* |
|
Height (cm) |
164.4 |
2.3 |
164.1 |
1.7 |
0.90 |
|
Body Mass (kg) |
60.5 |
2.9 |
60.1 |
2.7 |
0.93 |
|
Fat Free Mass (kg) |
45.9 |
1.7 |
43.1 |
1.5 |
0.24 |
|
Fat % |
23.7 |
1.5 |
28.3 |
1.9 |
0.04* |
|
Waist to Hip Ratio |
0.73 |
0.0 |
0.80 |
0.0 |
0.005* |
|
Blood Glucose (mg/dL) |
83.3 |
2.0 |
89.5 |
2.5 |
0.13 |
|
Total Cholesterol (mg/dL) |
167.7 |
9.6 |
183.5 |
7.6 |
0.22 |
|
LDL-C (mg/dL) |
100.8 |
9.5 |
97.0 |
5.6 |
0.73 |
|
HDL-C (mg/dL) |
59.8 |
7.0 |
66.8 |
4.4 |
0.41 |
|
Triglycerides (mg/dL) |
65.7 |
10.5 |
80.6 |
13.3 |
0.39 |
|
FFM-Adjusted 1RM Index |
7.89 |
0.5 |
6.53 |
0.3 |
0.026* |
|
FSH (mIU/mL) |
5.2 |
0.9 |
58.0 |
8.0 |
<0.0001* |
|
17β-estradiol (pg/mL) |
109.8 |
13.5 |
17.7 |
4.9 |
0.001* |
|
Data are presented as mean ± standard error. LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, FFM: fat free mass, 1RM: one repetition maximum, FSH: follicle stimulating hormone. FFM-Adjusted 1RM index was calculated by dividing an individual’s raw total (kg) by their fat free mass (kg). *P<0.05 between groups. |
|||||

References
- Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM et al. (2009) Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183:1393-1402. [Crossref]
- Stefanska A, Bergmann K, Sypniewska G (2015) Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv Clin Chem 72: 1-75. [Crossref]
- Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116: 1186-1194. [Crossref]
- Simpson ER (2003) Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86: 225-230. [Crossref]
- Dinarello CA (2000) Proinflammatory cytokines. Chest 118: 503-508. [Crossref]
- Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 80: 1055-1081. [Crossref]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29: 71-109. [Crossref]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M et al. (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908: 244-254. [Crossref]
- Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C et al. (1999) Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation 100: 717-722. [Crossref]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ et al. (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334-1359. [Crossref]
- Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35: 339-361. [Crossref]
- Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ et al. (2012) Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc 44: 2099-2110. [Crossref]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P et al. (2012) Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 97: 1159-1168. [Crossref]
- Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA et al. (2002) IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283: E1272- E1278. [Crossref]
- Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12: 6-33. [Crossref]
- Prestes J, Shiguemoto G, Botero JP, Frollini A, Dias R et al. (2009) Effects of resistance training on resistin, leptin, cytokines, and muscle force in elderly post-menopausal women. J Sports Sci 27: 1607-1615. [Crossref]
- Ihalainen J, Walker S, Paulsen G, Hakkinen K, Kraemer WJ et al. (2014) Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts. Eur J Appl Physiol 114: 2607-2616. [Crossref]
- Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL (2010) Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc 42: 314-325. [Crossref]
- Rutz S, Ouyang W (2016) Regulation of Interleukin-10 expression. Adv Exp Med Biol 941: 89-116. [Crossref]
- Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515: 287-291. [Crossref]
- Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K et al. (2005) Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 95: 514-521. [Crossref]
- Fatouros I, Chatzinikolaou A, Paltoglou G, Petridou A, Avloniti A et al. (2010) Acute resistance exercise results in catecholaminergic rather than hypothalamic-pituitary-adrenal axis stimulation during exercise in young men. Stress 13: 461-468. [Crossref]
- Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I et al. (2016) Load-specific inflammation mediating effects of resistance training in older persons. J Am Med Dir Assoc 17: 547-552. [Crossref]
- Pereira GB, Tibana RA, Navalta J, Sousa NM, Cordova C et al. (2013) Acute effects of resistance training on cytokines and osteoprotegerin in women with metabolic syndrome. Clin Physiol Funct Imaging 33: 122-130. [Crossref]
- Hirose L, Nosaka K, Newton M, Laveder A, Kano M et al. (2004) Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev 10: 75-90. [Crossref]
- Izquierdo M, Ibanez J, Calbet JA, Navarro-Amezqueta I, Gonzalez-Izal M et al. (2009) Cytokine and hormone responses to resistance training. Eur J Appl Physiol 107: 397-409. [Crossref]
- Jajtner AR, Hoffman JR, Gonzalez AM, Worts PR, Fragala MS et al. (2015) Comparison of the effects of electrical stimulation and cold-water immersion on muscle soreness after resistance exercise. J Sport Rehabil 24: 99-108. [Crossref]
- Wells AJ, Hoffman JR, Jajtner AR, Varanoske AN, Church DD et al. (2016) Monocyte recruitment after high-intensity and high-volume resistance exercise. Med Sci Sports Exerc 48: 1169-1178. [Crossref]
- Kovats S (2015) Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63-69. [Crossref]
- Khan D, Ansar Ahmed S (2015) The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 6: 635. [Crossref]
- Bachy V, Williams DJ, Ibrahim MA (2008) Altered dendritic cell function in normal pregnancy. J Reprod Immunol 78: 11-21. [Crossref]
- Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A et al. (2002) Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res 70: 238-248. [Crossref]
- Matalka KZ (2003) The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett 24: 185-191. [Crossref]
- Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D et al. (2001) Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol 79: 400-406. [Crossref]
- Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A (2015) Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep 5: 15224. [Crossref]
- Wells JC (2007) Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 21: 415-430. [Crossref]
- Purohit A, Reed MJ (2002) Regulation of estrogen synthesis in postmenopausal women. Steroids 67: 979-983. [Crossref]
- Barakat R, Oakley O, Kim H, Jin J, Ko CJ (2016) Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep 49: 488-496. [Crossref]
- Simpson ER (2002) Aromatization of androgens in women: current concepts and findings. Fertil Steril 77: S6-S10. [Crossref]
- Mattsson C, Olsson T (2007) Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem 14: 2918-2924. [Crossref]
- Bowers LW, Brenner AJ, Hursting SD, Tekmal RR, deGraffenried LA (2015) Obesity-associated systemic interleukin-6 promotes pre-adipocyte aromatase expression via increased breast cancer cell prostaglandin E2 production. Breast Cancer Res Treat 149: 49-57. [Crossref]
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C et al. (2011) Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 4: 329-346. [Crossref]
