Journals
Management of uncontained bone voids with Calcium Sulphate Ceramic (Modified Masquelet / Modified AMIC with Cerament Bio-Composite) Case report and review of literature
A B S T R A C T
Introduction: Large traumatic bone voids are challenging to treat. Autografts are associated with donor site morbidity and limited availability. Bone graft substitutes are successful alternative to fill bone voids.
Case Presentation: The management of two patients with an open tibial fracture with segmental bone loss and other patient with periarticular calcaneal void associated with chondral loss.
Treatment and outcomes: For first case, a contained cavity was made using Septocoll E, an absorbable collagen fleece, to mimic a pseudo-membrane using Masquelet-technique. Bone void was filled with Cerament-G and autologous bone graft. Second case with large peri-articular calcaneal void and chondral loss, Cerament-G and autologous bone graft were used, and articular defect reconstruction was done with synthetic chondral tissue. Both patients had painless mobility and consolidation of bone void.
Discussion: We represent two exceptional cases of traumatic bone void which were treated with modified masquelet/modified AMIC with Cerament Bio-Composite with satisfactory outcomes.
Keywords
Large bone void, cerament G, chondrotissue, septocoll E, traumatic chondral loss
Introduction
Traumatic segmental bone loss and large periarticular bone voids are clinically challenging to treat with significant long-term morbidity. Although smaller defects can be successfully treated with autologous cancellous bone grafting, larger defects require more complex alternatives like, distraction osteogenesis, vascularised fibular grafting, allografts or fibular pro-tibia grafting. This report aims highlights two patients with large uncontained bone voids treated with a calcium sulphate hydroxyapatite bio composite layered with cancellous bone. Novel techniques to achieve containment of an uncontained defect is discussed.
Case Report
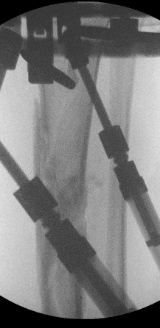
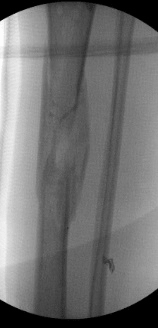
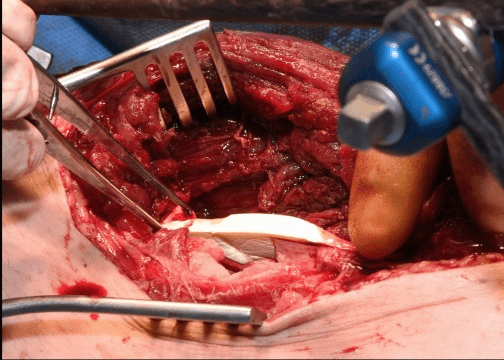
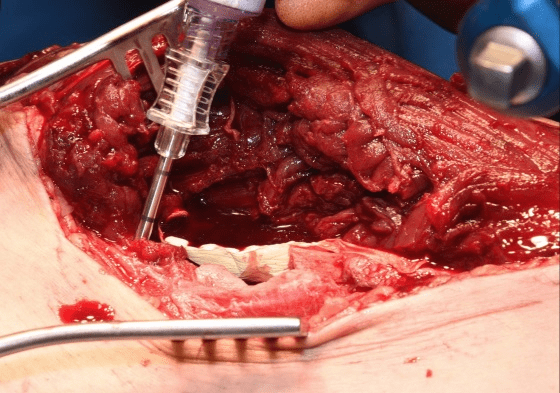
Case A is a 47-y old lady who was involved in blast injury from an improvised explosive device. She experienced penetrating shrapnel injuries to both lower limbs with an open Gustillo-Anderson Grade 3a injury to left tibial shaft. CT scan performed after resuscitation which showed large void in shaft of left tibia. Damage control surgery was performed initially involving spanning external fixator and fasciotomy. The injury resulted in a segmental bone 5cm x 2cm with circumferential bone loss (Figure 1). On this occasion, after through debridement the uncontained cavity was converted into contained cavity by using Septocoll E, an absorbable equine collagen fleece, to mimic a pseudo membrane as in Masquelet technique (induced membrane). The bone void was now filled with bone substitute ceramic bio-composite (Cerament G), layered alternating with autologous cancellous bone graft (Figure 2). Circular frame was applied and patient allowed to weight bear. At 12 months she had pain free mobility with complete consolidation of bone void.
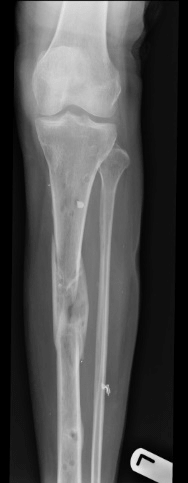
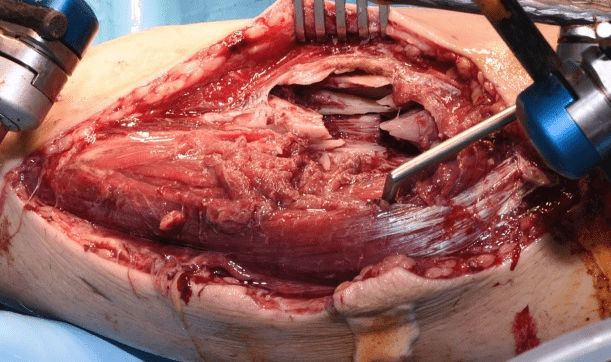
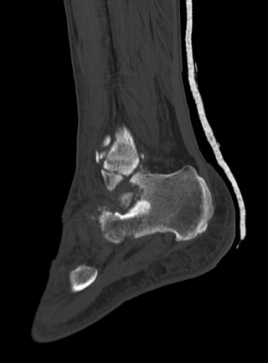
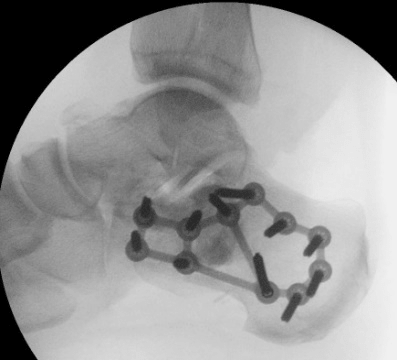
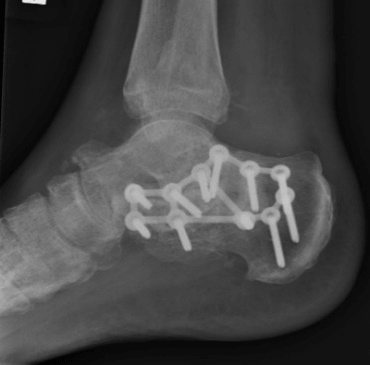
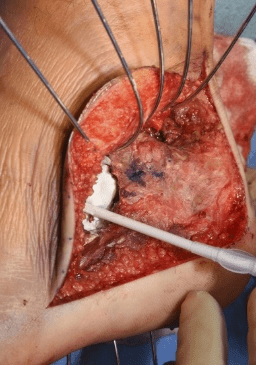
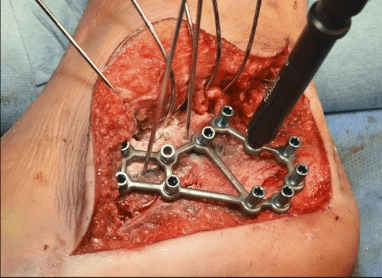
Case B is 56 years old male having large periarticular void with comminuted fracture of calcaneum involving talocalcaneal joint after history of fall. A large void in body of calcaneum was confirmed on CT scan (Figure 3) with loss of the subtalar joint and its chondral surface. Open reduction and internal fixation was done by lateral approach (Figure 4). Bone void was filled with multiple layers of Cerament G and cancellous bone graft. The articular defect was reconstructed with a synthetic chondral tissue (Chondrotissue). Partial weight bearing was started at 6 weeks and full weight bearing at 12 weeks. At final follow up after 6 months, union was confirmed by CT scan and patient has painless mobility with no joint collapse.
Figure 1: Radiographic images of Case A: a. Pre-operative fluoroscopy showing void in tibial diaphysis b. After application of CeramentG c. At 10 months d. Final radiograph at 12 months
Figure 2: Operative images of Case A: a. Large void visible in tibial shaft b. Septocoll E application in the void c. Cerament G application in the void
Figure 3: Radiographic images of Case B: a. Pre-operative CT of left ankle showing intra articular defect in calcaneum. b. Fluoroscopy image taken during procedure showing lateral plate on calcaneum c. Final radiograph taken at 6 months
Figure 4: Operative images of Case B: a. Lateral view of left foot showing void in calcaneum after elevating the lateral flap b. Chondrotisse used for articular surface reconstruction c. Bone void filled with Cerament G d. Application of plate on calcaneum
Discussion
Management of large defects depends on surgeon experience and training. Autologous bone graft has principal limitations of donor site morbidity and a limited availability. The ideal bone graft substitute must contain osteoconductive matrix, osteoinductive proteins, and osteogenic cells but most can provide some, but not all, of these elements. The major categories of bone graft substitutes include allograft bone, demineralized bone matrix (DBM), nonorganic synthetic void fillers and bioactive ceramics, and bone morphogenetic proteins. Cerament G is an absorbable bio-composite containing hydroxyapatite particle embedded in calcium sulphate carrier and gentamicin as an antibiotic. The compressive strength provided by this injectable bio-ceramic is like that of cancellous bone [1]. Cerament G has been used successfully used in our department as void filler for treatment of chronic osteomyelitis and open Gustilo Anderson type IIIB fractures [2].
The Case A has Gustilo Anderson type IIIB fracture of tibial diaphysis with uncontained large bone void. During definitive procedure multiple layers of Cerament G and cancellous bone graft from iliac crest was used to fill up the cavity. Containment of cavity is pre requisite for the bone graft to function which is provided by Septocoll E, an absorbable equine collagen fleece. This is one stage procedure which a modified version of Masquelet induced membrane technique. This was first proposed by Masquelet et al. in 1980 in which polymethylmethacrylate (PMMA) spacer was implanted for 6 to 8 weeks in the segmental cortical bone defect and an induction membrane is formed surrounding the defect, containing osteogenic and osteoinductive factors [3]. During second stage PMMA spacer is removed and fills the defect with autologous bone graft. This induction membrane serves as an envelope, encapsulating the bone graft and promoting bone healing. The induced membrane creates a contained cavity thus limiting autograft resorption. Masquelet two-stage procedure has the drawback of second procedure of removal of the PMMA spacer. To replace the PMMA spacer, Yi-Hsun Yu and colleagues has used biodegradable nanofibers in Masquelet procedure for treatment of segmental bone defects in animals [4]. We successfully used Septocoll E, an absorbable equine collagen fleece to work as pseudo membrane which has not been previously reported.
The second case in our case report has large bone void with chondral loss in calcaneum involving talocalcaneal and calcaneocuboid joint. In addition to bone voids, cartilage defects are of particular interest for the orthopaedic surgeon as these have limited capacity to heal after injury.Recently, one-step cartilage repair procedures have been developed for the treatment of chondral defects using autologous matrix-induced chondrogenesis (AMIC) [5]. These procedures utilize subchondral progenitor or stem cells, which are released into the defect by bone marrow stimulation, and different types of resorbable scaffolds that may help to keep the cells and the newly formed tissue in the defect [6]. The use of synthetic, polyglycolic acid hyaluronane implant (chondrotissue®) for the treatment of full-thickness cartilage defects of the knee has been used by Siclari A. and colleagues [6]. Chondrotissue is a cell free, resorbable polymer-based textile scaffold, which provides initial mechanical stability and optimal environment for mesenchymal stem cells to regenerate [7]. We were successful in managing the intraarticular bone defect and cartilage loss by using Cerament G mixed with autologous bone graft supplemented with on-lay chondrotissue scaffold. This novel technique enabled salvage of the joint with both articular and chondral reconstruction. We would recommend both these techniques (modified masquelet / modified AMIC with Cerament Bio-Composite) in the management these challenging injuries.
Acknowledgment
The authors declare that no part of this study has been taken from existing published or unpublished materials without due acknowledgement and that all secondary materials used herein has been fully referenced. We declare that this work received no financial support of any source.
Contributions
Noman Shakeel Niazi Ahmed Aljawadi Mazin Al-salihy Anand Pillai
Article Info
Article Type
Case Report & Review of LiteraturePublication history
Received: Thu 01, Aug 2019Accepted: Fri 16, Aug 2019
Published: Fri 30, Aug 2019
Copyright
© 2023 Noman Shakeel Niazi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2019.04.07
Author Info
Ahmed Aljawadi Mazin Al-salihy Anand Pillai Noman Shakeel Niazi
Corresponding Author
Noman Shakeel NiaziTrauma and Orthopaedics, Manchester Foundation Trust, Southmoor Rd, Wythenshawe, Manchester, M23 9LT, The United Kingdom
Figures & Tables



References
- Nilsson M, Wang JS, Wielanek L, Tanner KE, Lidgren L (2004) Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br 86: 120-125. [Crossref]
- Jahangir N, Niazi N, Aljawadi A, Reid A, Wong J et al. (2019) The use of adjuvant local antibiotic hydroxyapatite bio-composite in the management of open Gustilo Anderson type IIIB fractures. A prospective review. J Orthop 16: 278-282. [Crossref]
- Masquelet AC, Fitoussi F, Begue T, Muller GP (2000) Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthét 45: 346-353. [Crossref]
- Yi-Hsun Yu, Ren-Chin Wu, Demei Lee, Che-Kang Chen, Shih-Jung Liu (2018) Artificial Membrane Induced by Novel Biodegradable Nanofibers in the Masquelet Procedure for Treatment of Segmental Bone Defects.Hindawi. J Nanomate 2018: 1-8.
- Benthien JP, Behrens P (2011) The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc 19: 1316-1319. [Crossref]
- Siclari A, Mascaro G, Kaps C, Boux E (2014) A 5-Year Follow-Up After Cartilage Repair in the Knee Using a Platelet- Rich Plasma-Immersed Polymer-Based Implant. Open Orthop J 8: 346-354. [Crossref]
- 7.Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M et al. (2007) Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials 28: 5570-5580. [Crossref]
