Management of Spontaneous Cerebrospinal Fluid Leaks in the Middle Cranial Fossa and Bone Tegmen Tympani Defect in Patients with Meningitis and Rhinorrhea
A B S T R A C T
The pathogenetic process of spontaneous CerebroSpinal Fluid (CSF) leaks in the middle cranial fossa has not been clearly identified yet. It is related to a tegmen defect associated to the presence of a simultaneous encephalocele or meningoencephalocele. The main complication of a CSF leak is meningitis, whose occurrence rate ranges from 4% to 50% according to different causes and conditions of the leak [1]. Surgical approaches to temporal bone reconstruction include middle cranial fossa (MCF) craniotomy, transmastoid (TM), or a combined (MCF/TM) approach. In our experience, we describe 2 cases of patients who presented with CSF rhinorrhea and meningoencephaloceles correlated with conductive hearing loss and meningitis. The MCF approach is a considerable way to successful repair CSF leaks and encephaloceles due to tegmen tympani and dural defects.
Keywords
Spontaneous cerebrospinal fluid leaks, MRI, high resolution tomography scan, hydroxyapatite, middle cranial fossa, transmastoid
Introduction
Cerebrospinal fluid (CSF) leak can be acquired or spontaneous. Acquired cerebrospinal fluid leaks can be secondary to various disorders, including tumors, traumatic injuries and infectious-inflammatory conditions (otitis media, cholesteatoma) or to iatrogenic damage, such as an irradiation therapy. On the other hand, spontaneous CSF leaks are less common and have uncertain pathogenetic mechanisms, although it is described that obesity and idiopathic. Intracranial hypertension are associated to spontaneous CSF leaks [2]. Indeed, increased intracranial pressure could create an erosion of anterior and middle cranial fossa, developing bone defect, dural exposure and the risk of CSF leak occurrence. Atypical arachnoid granulations and genetic thinning of tegmen tympani are less frequent mechanisms involved in the development of leakage [3, 4]. CSF leak typically presents with rhinorrhea, otorrhea, headache, hearing impairment and recurrent meningitis [5]. Seizures or neurological deficits are additional consequences, especially in case of encephalocele or meningoencephalocele [6]. Meningitis is the most dangerous and common complication. Despite antibiotic therapy, the mortality rate from bacterial meningitis in adults is about 33% [1]. Surviving patients can be affected by severe morbidity, such as encephalopathy, seizure disorders and cranial nerves deficits. The elevated risk of life-threatening complications remarks the importance of early detection, accurate diagnosis, and suitable repair of CSF leaks.
Case 1
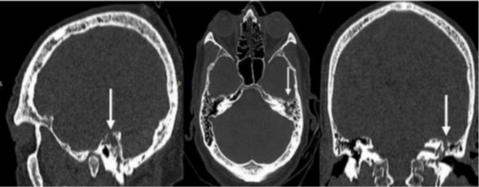
A 75-year-old man was admitted to the Emergency Department of Umberto I Hospital with sudden high fever, severe headache, nausea with vomiting, lethargy and rigor nucalis. Then, he was admitted to the infectious disease department and treated with an antibiotic therapy for three weeks (ceftriaxone 4 g/day). The patient’s recovery was complicated by an acute coronary syndrome treated with coronary angioplasty and antiplatelet therapy. After a period of 6 weeks bed rest, when patient restarted mobilization in sitting and standing position, immediately reported CSF leakage and increased flow of nasal discharge while was standing. Brain and cranial computed tomography (CT) scan of the patient showed bony defect in the roof of the left petrous pyramid in the tegmen tympani area associated to a slight meningoencephalocele. Furthermore, the petrous bone appeared iso-hyperdense due to the presence of fluid material in air cells (Figure 1). A brain MRI scan showed a cavity filled with CSF-like fluid with reactive gliosis at the level of the inferior temporal gyrus. This cavity appeared contiguous to the roof of the mastoid, filled by CSF-like signal fluid material, in accordance with the erosion reported in the CT scan (Figure 2).
Figure 1: CT scan result of 11yrs girl crash car injured. At the time of the crash, the girl was restraint by lap seat belt.
Figure 2: A brain MRI scan showing brain atrophy as A) a cavity filled with CSF-like intensity fluid (T2) and B) MPR weighted images.
Figure 3: Surgical procedure: A) Identification of the fistula point (black arrows) B) Wax apposition C) Fibrin glue apposition D) Autologous fat graft.
The patient was referred to the neurosurgery ward for neurosurgical repair treatment. After a wide discussion about the surgical strategy, a middle cranial fossa approach was performed. The surgical steps include a left temporal flap and craniotomy and a subtemporal extradural approach allowing the identification of the bone defect on the surface of petrous pyramid. At the same level a dural defect and a small encephalocele were recognized. The dura mater was repaired and the bone defect was filled with bone wax apposition. Autologous fat, fibrin glue (tissucol) and activated polyethylene glycol and polyethyleneimine (Adherus dural sealant) were used in multilayer technique to seal off the fistula (Figure 3). After 48 hours, the patient presented a single partial epileptic seizure episode, treated with pharmacological therapy (levetiracetam 500 mg twice a day). After 7 days, the patient was discharged in good clinical and neurological conditions with no evidence of CSF leak. At 1-year follow-up, the patient did not present rhinoliquorrhea and seizures. The follow-up MRI showed, at the level of the mastoid roof, the presence of hyperintense T2 tissue, to be referred to surgical filling material and a slight reduction in CSF-like fluid material filling of the left mastoid cells (Figure 4).
Figure 4: Post-operative MRI showing surgical filling material and a slight reduction in CSF-like fluid material filling of the left mastoid cavity.
Case 2
An 81-year-old patient was admitted to Emergency Department of Umberto I Hospital for recurrent episodes of rhinorrhea occurring under specific conditions such as flexion of the head and coughing. The patient referred two previous operations, performed in another hospital, the first one to repair a spontaneous middle cranial fossa CSF leak and the second one to evacuate a post-surgical left temporal haemorrhage. Head CT scan showed a bony defect in the roof of the left petrous pyramid. MRI showed a large portion of inflammatory tissue involving the left mastoid cells with partial involvement of the middle ear. Moreover, a smaller amount of inflammatory tissue in the controlateral right mastoid and a CSF-like signaled cavity (post-surgical malacic area) in continuity with the temporal horn of the ipsilateral lateral ventricle were evident (Figures 5 & 6). The patient was transferred to Neurosurgery ward to plan the surgical treatment. During the examination, the patient didn’t present any symptoms and signs, except for a copious rinoliquorrhea during head flexion. The audiometry test showed a severe hearing impairment. A subtemporal extradural approach was performed in order to reach the bone defect. First, the bone defect was identified and closed by bone wax apposition. Then, dura mater was repaired. Autologous fat, fibrin glue (tissucol) and activated polyethylene glycol and polyethyleneimine (Adherus dural sealant) were used in multilayer technique to seal off the fistula.
The patient was transferred to Intensive Care Unit for 24 hours for postoperative observation. On the second postoperative day, some episodes of rhinoliquorrhea occurred. Therefore, the patient was treated with lumbar drain and supine position for 4 days. Later, when he was again in standing position, a single episode of a small amount of CSF leak occurred. The patient underwent head CT and MRI scan, that did not show further fistula points. Considering the possibility that these post-operative rhinoliquorrhea episodes were due to the residual amount of CSF in the mastoid cells, we decided for a wait and see strategy. At discharge from Neurosurgery ward, rhinoliquorrhea was absent. At 1-year follow-up, the patient did not show or reported presence of CSF leakage and the audiometry test was unchanged. The follow-up MRI showed in the mastoid roof some hyperintense T2 tissue, to be referred to surgical filling material, and the reduction of the CSF-like signalling fluid in the left mastoid cells (Figure 7).
Figure 5: Preoperative MRI showing A) the previous left temporal surgical site, in continuity with the temporal horn of the lateral ventricle; B) the presence of fat-like signaled subcutaneous content with a small CSF-like component; C) Inflammatory tissue in the left mastoid cells.
Figure 6: T2 sequences (pre-operative MRI) showing the fistula point.
Figure 7: Post-operative MRI showing reduced subcutaneous fluid content, but persistence of inflammatory tissue in the mastoid cells (especially in the left mastoid cell).
Discussion
There is not a clear definition for "spontaneous CSF (SCSF) leak". Many authors define SCSF leak when there are not identifiable causes, while other authors identify it when there is not a clear correlation with trauma, infections or tumors [7]. SCSF leak may be classified according to the site or according to the known or presumed cause. In 1999, Har-El classified CSF leakage according to position and to the traumatic or non-traumatic etiology. He also divided traumatic SCSF in surgical and nonsurgical csf leaks and “non traumatic” SCSF in high pressure leaks and normal pressure leaks [8]. However, an accurate investigation through radiological exams, endoscopic procedures and surgery will frequently result in the identification of a probable cause of the leak. Therefore, most of these "idiopathic" cases will eventually have a more specific diagnosis. Three most relevant theories about the pathogenesis of SCSF leak are reported in literature.
The first one attributes the occurrence of SCSF leak to tegmen tympani atrophy, due to an erosion caused by the physiological CSF pulse [9]. Several studies point out the relationship between spontaneous CSF leaks and obesity [10-13]. The precise mechanism through which obesity contributes to SCSF leak has not been completely explained. According to the most credited theories, elevated body mass index (BMI) would lead to an increased venous pressure and a decreased venous return from the brain, this resulting in intracranial hypertension and consequent CSF outflow from the arachnoid villi into the dural sinuses. Gacek et al. reported another pathogenetic theory based on the presence of middle cranial fossa abnormal arachnoid granulations: during embryonic development, some arachnoid granulations can form directly on the bone surface without interposing endothelial sinus wall [14]. If these granulations expand, they could eventually erode temporal bone, establishing a communication between CSF space and mastoid air cells. Gacek et al. identified an 8.5% incidence of arachnoid granulations in the posterior fossa of the temporal bone, while Ferguson et al. observed an incidence of 22% of pit holes created by arachnoid granulations in the middle fossa surface [15].
The last etiopathogenetical theory about the development of SCSF leaks is connected with an excessive process of pneumatisation during the development of temporal bone which leads to the bone surface thickening [16]. Patients with spontaneous CSF leak of temporal bone often present with middle ear pathology, such as serous otitis, otorrhea, imbalance, tinnitus or hearing loss, but they can also present with only headaches and rhinorrhea. The most feared complication is meningitis, which may lead to death or to life impairing neurological consequences. Other late severe complications, include meningoencephalocele and encephalocele, leading to intracranial abscesses and seizures. Furthermore, features of intracranial hypertension may occur, such as papilledema, visual deficit, memory loss and ventricle dilatation. One useful diagnostic study to detect CSF leaks is testing for beta-2 trasferrin, a characteristic CSF biomarker [17]. If it results positive, the second level exam is high resolution cranial CT scan, which can identify skull base defects with specificity and sensitivity up to 88-95% [18]. Furthermore, the surgeon can utilize CT scan in a cerebral navigation system to plan best surgical approach.
However, the skull base may present many defects on its own surface: in this situation, it is difficult to identify the origin of the CSF leak [19]. Head-MRI is important to study the cerebral tissue and useful to show herniation of the dura mater into the temporal bone. A high signal on the T2 weighted images in the mastoid cavities confirms CSF leakage. Moreover, it is helpful to set up a differential diagnosis among the several pathologies of the patient. HRCT and MRI cerebral scans combined results may help to find the cause of spontaneous CSF leak and to plan the correct therapeutic process.
Three surgical approaches can be used to repair the SCSF leaks of temporal bone: transmastoid approach, subtemporal approach and combined approach. The choice between these approaches depends on size and location of the defect, clinical status, hearing status and surgeon’s experience. Many authors recommend to perform transmastoid approach to repair the skull base defect of posterior fossa and subtemporal approach to repair tegmen defects [20]. The subtemporal extradural approach is the most commonly used in literature. It prevents hearing impairment and a wide exposure of middle cranial fossa including the anterior part of the temporal bone. Frequently, a clinical evidence of SCSF leaks are caused by multiple bone defects (that cannot be always completely detected on CT or MRI head scan), so a wider exposure of middle cranial fossa can help detecting previously unknown bone defects [16].
The subtemporal approach consists of performing an incision of the temporalis muscle to expose the temporalis squama. Temporal lobe is exposed performing a 4 cm x 4 cm craniotomy. Then a slight temporal lobe retraction, combined with a correct head fixation, is mandatory to show the superior portion of the petrous bone in order to expose the tegmental defect, so that it is possible to perform dural repairing and to appose some grafts between the bony defect and the dura. According to many authors, MCF approach is often used for all bony defects, but they recommend taking into consideration the possible complications. Seizures, brain swelling, venous infarction and neurological deficits are the most dangerous pitfalls about this approach [16, 21, 22]. The transmastoid approach consists of a retroauricolar skin incision and a mastoidectomy. It is an extracranial exposure of middle and posterior cranial fossa, which avoids brain tissue manipulation. The mastoid cavity is filled with autologous or heterologous materials to close the leakage. The ossicular chain can frequently be damaged. Usually, this approach is used when patients present with otorrhea and hearing loss. Alternatively, it is used to treat small defects of posterior cranial fossa [23].
Combined approach helps surgeons to obtain a wider surgical field including both middle and posterior cranial fossa. Furthermore, this approach is useful to treat recurrent CSF leaks and/or in presence of multiple bone defects [24]. Both the transmastoid and the subtemporal approach could present a risk of CSF leak recurrence, especially in case of large bone defects associated with meningoencephalocele. Leonetti et al. proposed, in two cases of recurrent CSF leak after a middle cranial fossa craniotomy, a subtotal petrosectomy with eustachian tube, middle ear and mastoid obstruction [25].
In our series, a multilayer grafts technique is used to seal the defect. Several kinds of grafts are described in literature [1, 15, 20]. The material of the graft can be autologous, such as abdominal fat, fascia lata, galea, cortical bone, temporalis muscle, or heterologous, such as bovine dura. Then, it is valuable to use eterologous biocompatible materials, such as bone wax, and fibrin glue. Among the various eterologous materials, Kveton et al. described the largest series of temporal bone defects sealed with hydroxyapatite (HAC), encounting 102 patients. In this series, the 97% of SCSF leaks were successfully treated except one case of recurrence. The main complication of HAC use is the post-operative infection incidence (5% of the treated cases) [26].
About the use of lumbar drain in surgically treated SCSF leaks, it is quite controversial. Nelson et al., retrospectively analysed the perioperative use of lumbar drain in 60 patients with SCSF leaks due to tegmen tympani defects. The effectiveness of surgical treatment was not significantly different in patients who underwent to lumbar drain and patients who did not. On the other hand, lumbar drain resulted in increased hospital cost due to the length of stay and infections incidence [27]. According with these authors, we support the theory that lumbar drain should be used only in selected cases, especially in patients with post-surgical rhinoliquorrea occurrence, and only for a short period (3-5 days). In case of persistent rhinoliquorrea after lumbar drain removal, a surgical revision should be considered.
Conclusion
In our experience, all patients presenting a spontaneous CSF leak should be carefully studied with high-resolution CT and MRI head scan, to rule out the presence of a bone defect of the petrous pyramid. We recommend the surgical repair through a middle cranial fossa approach, since it provides a wider exposure and a good control of the operative field, ensuring a better sealing of the fistula, with low recurrence rate. Moreover, in patients who do not present with hearing loss, this approach avoids hearing impairment, which is more common with transmastoid approach.
Article Info
Article Type
Case ReportPublication history
Received: Fri 24, Jan 2020Accepted: Sat 15, Feb 2020
Published: Thu 27, Feb 2020
Copyright
© 2023 Francesco Paglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.02.09
Author Info
Altamura C.F D'Angelo L Francesco Paglia Marzetti F Sampirisi L Santoro A
Corresponding Author
Francesco PagliaDepartment of Neurology and Psychiatry, Neurosurgery, Sapienza University of Rome, Rome, Italy
Figures & Tables






References
- Savva A, Taylor MJ, Beatty CW (2003) Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope 113: 50-56. [Crossref]
- Quatre R, Attye A, Righini CA, Reyt E, Giai J et al. (2017) Spontaneous Cerebrospinal Fluid Rhinorrhea: Association with Body Weight and Imaging Data. J Neurol Surg B Skull Base 78: 419-424. [Crossref]
- Gacek RR (1990) Arachnoid granulation cerebrospinal fluid otorrhea. Ann Otol Rhinol Laryngol 99: 854-862. [Crossref]
- Psaltis AJ, Overton LJ, Thomas WW 3rd, Fox NF, Banks CA et al. (2014) Differences in skull base thickness in patients with spontaneous cerebrospinal fluid leaks. Am J Rhinol Allergy 28: e73-e79. [Crossref]
- Rao N, Redleaf M (2016) Spontaneous middle cranial fossa cerebrospinal fluid otorrhea in adults. Laryngoscope 26: 464-468. [Crossref]
- Hubbard JL, McDonald TJ, Pearson BW, Laws ER Jr (1985) Spontaneous cerebrospinal fluid rhinorrhea; evolving concepts in diagnosis and surgical management based on the Mayo Clinic experience from 1970 through 1981. Neurosurgery 16: 314-321. [Crossref]
- Pappas DG, Pappas DG, Hoffman RA, Harris SD (1996) Spontaneous cerebrospinal fluid leaks originating from multiple skull base defects. Skull Base Surg 6: 227-230. [Crossref]
- Har-El G (1999) What is "spontaneous" cerebrospinal fluid rhinorrhea? Classification of cerebrospinal fluid leaks. Ann Otol Rhinol Laryngol 108: 323-326. [Crossref]
- Ommaya AK (1964) Cerebrospinal fluid rhinorrhea. Neurology 14: 106-113. [Crossref]
- Son HJ, Karkas A, Buchanan P, Giurintano JP, Theodosopoulos P et al. (2014) Spontaneous cerebrospinal fluid effusion of the temporal bone: repair, audiological outcomes, and obesity. Laryngoscope 124: 1204-1208. [Crossref]
- Stevens SM, Lambert PR, Rizk H, McIlwain WR, Nguyen SA et al. (2015) Novel radiographic measurement algorithm demonstrating a link between obesity and lateral skull base attenuation. Otolaryngol Head Neck Surg 152: 172-179. [Crossref]
- Stucken EZ, Selesnick SH, Brown KD (2012) The role of obesity in spontaneous temporal bone encephaloceles and CSF leak. Otol Neurotol 33: 1412-1417. [Crossref]
- Vivas EX, Mccall A, Raz Y, Fernandez Miranda JC, Gardner P et al. (2014) ICP, BMI, surgical repair, and CSF diversion in patients presenting with spontaneous CSF otorrhea. Otol Neurotol 35: 344-347. [Crossref]
- Gacek RR, Gacek MR, Tart R (1999) Adult spontaneous cerebrospinal fluid otorrhea: diagnosis and management. Am J Otol 20: 770-776. [Crossref]
- Ferguson BJ, Wilkins RH, Hudson W, Farmer J Jr (1986) Spontaneous CSF otorrhea from tegmen and posterior fossa defects. Laryngoscope 96: 635-644. [Crossref]
- Merchant SN, McKenna MJ (2000) Neurotologic manifestationsand treatment of multiple spontaneous tegmental defects. Am J Otol 21: 234-239. [Crossref]
- Bachmann Harildstad G (2008) Diagnostic values of beta-2 transferrin and beta-trace protein as markers for cerebrospinal fluid fistula. Rhinology 46: 82-85. [Crossref]
- Reddy M, Baugnon K (2017) Imaging of Cerebrospinal Fluid Rhinorrhea and Otorrhea. Radiol Clin North Am 55: 167-187. [Crossref]
- O'Connell BP, Hunter JB, Sweeney AD, Thompson RC, Chambless LB et al. (2017) Outcomes of the Suture "Pull-Through" Technique for Repair of Lateral Skull Base CSF Fistula and Encephaloceles. Otol Neurotol 38: 416-422. [Crossref]
- Cakir Cetin A, Karabay N, Guneri EA (2017) Our experience in the management of CSF otorrhea: A transmastoid approach with middle ear cavity obliteration and a middle cranial fossa approach. Turk Neurosurg. [Crossref]
- Sönmez S, Şahin B, Polat B, Çomoğlu Ş, Orhan KS (2017) Repair of Tegmen Tympani Defect Presenting with Spontaneous Cerebrospinal Fluid Otorrhea Using the Middle Cranial Fossa Approach. J Int Adv Otol 13: 430-433. [Crossref]
- Braca JA 3rd, Marzo S, Prabhu VC (2013) Cerebrospinal Fluid Leakage from Tegmen Tympani Defects Repaired via the Middle Cranial Fossa Approach. J Neurol Surg B Skull Base 74: 103-107. [Crossref]
- Marchioni D, Bonali M, Alicandri Ciufelli M, Rubini A, Pavesi G et al. (2014) Combined approach for tegmen defects repair in patients with cerebrospinal fluid otorrhea or herniations: our experience. J Neurol Surg B Skull Base 75: 279-287. [Crossref]
- Patel RB, Kwartler JA, Hodosh RM, Baredes S (2000) Spontaneous cerebrospinal fluid leakage and middle ear encephalocele in seven patients. Ear Nose Throat J 79: 372-373, 376-378. [Crossref]
- Leonetti JP, Marzo S, Anderson D, Origitano T, Vukas DD (2005) Spontaneous transtemporal CSF leakage: a study of 51 cases. Ear Nose Throat J 84: 700, 702-704, 706. [Crossref]
- Kveton JF, Coelho DH (2004) Hydroxyapatite cement in temporal bone surgery: a 10 year experience. Laryngoscope 114: 33-37. [Crossref]
- Nelson RF, Roche JP, Gantz BJ, Hansen MR (2016) Middle Cranial Fossa (MCF) Approach Without the Use of Lumbar Drain for the Management of Spontaneous Cerebral Spinal Fluid (CSF) Leaks. Otol Neurotol 37: 1625-1629. [Crossref]
