Management of Anterior Chamber Dislocation of a Fluocinolone Acetonide Implant: A Case Report
Management of Anterior Chamber Dislocation of a Fluocinolone Acetonide Implant: A Case Report
A B S T R A C T
Background: Fluocinolone acetonide implant (ILUVIEN) is a non-biodegradable cylindrical polyimide tube that is injected into the vitreous cavity. Migration to the anterior chamber can potentially occur, especially in patients with posterior capsular defects and vitrectomized eyes, although it is considered an uncommon complication. The best surgical technique is still unknown. We describe a simple technique for reinserting the migrated ILUVIEN implant in the posterior cavity without compromising its integrity.
Case Presentation: Under topical anaesthesia, a corneal clear beveled limbal incision is made with a 20G angled side port blade. Balanced saline solution is injected with a 27G anterior chamber cannula to mobilize the implant and a reverse sinskey hook is then used to push the implant to the vitreous cavity between the iris and the intraocular lens without the need of viscoelastic.
Conclusion: We report a simple and quick technique for surgical repositioning an ILUVIEN implant that required minimal manipulation and resulted in minimal tissue disturbance without compromising implant integrity and effectiveness. It is important to be cautious while using ILUVIEN in patients with capsular defects, zonular weakness, and previous vitrectomy. We recommend using a reverse sinskey hook as a smaller entry incision can be made to maintain the sealing of the anterior chamber.
Keywords
Iluvien, intravitreal implant, cystoids macular edema, anterior chamber dislocation, surgical technique
Background
The development of intraocular implants has revolutionized the treatment of retinal and macular diseases in light of the need of reliable sustained delivery systems to avoid repeated injections and fluctuations in steroid concentration. Among them, Ozurdex by Allergan Inc (Dublin, Ireland) and ILUVIEN by Alimera (GA, USA) are widely used and have significantly decreased the incidence of several visual loss caused by diabetic macular edema (DME), retinal vein occlusion and ocular inflammations [1].
Fluocinolone acetonide (FAc) implant is a non-biodegradable cylindrical polyimide tube, loaded with 190 µgr of FAc that is injected into the posterior cavity through a 25-gauge applicator in an outpatient setting. Major complications of ILUVIEN implant include glaucoma, conjunctival haemorrhage and induction or worsening of cataract. Migration of the Fac implant to the anterior chamber (AC) can potentially occur, especially in patients with posterior capsular defects and vitrectomized eyes, although it is considered an uncommon complication. Early removal of AC-dislocated FAc implant is key to avoid corneal edema and damage from raised intraocular pressure (IOP) [2, 3]. However, the best surgical technique is still unknown. Our purpose is to describe a simple technique for reinserting the AC-migrated FAc implant in the vitreous cavity without compromising its integrity.
Case Presentation
A 71-year-old female with a 15-year history of type 2 diabetes had diffuse DME diagnosed in her left eye in February 2012. Previous therapies included panretinal laser photocoagulation, macular grid laser and pro re nata intravitreal ranibizumab injections. In March 2017, the patient developed cataract and had a phacoemulsification with a posterior intraocular lens (IOL) complete dislocation onto the retina that required a vitrectomy and the implantation of a retropupillar iris claw lens. After surgery, recurrence of the macular edema was observed and Ozurdex was then administered with reduction in central macular thickness (CMT) and visual acuity (VA) improvement. The patient continued treatment with Ozurdex every 5 months and after 5 implants a new relapse of the macular edema was observed with CMT of 463µm and VA of 55 ETDRS letters. At this point, the macular edema was considered chronic and the patient was treated with intravitreal ILUVIEN which was performed without complication. One week after the procedure CMT decreased to 370 µm and VA improved to 60 ETDRS letters. IOP remained within normal limits.esenteric lymph-nodes and a 12 mm in diameter lesion in the VI-VII segment of the liver.
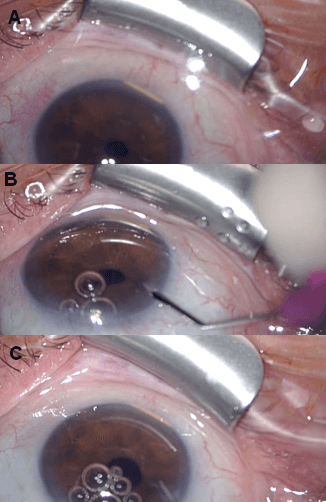
On Day 45 after the intravitreal injection, the patient presented to the Emergency Department with pain-less decreased vision of 44 ETDRS and the slit lamp examination revealed that the ILUVIEN had spontaneously migrated to the AC where it was lying horizontally in the inferior angle (Figure 1A) and causing mild inferior corneal edema. IOP was 22 mmHg. To prevent corneal decompensation, we opted for an implant repositioning.
Figure 1: A) Anterior segment examination showing the ILUVIEN implant in the anterior chamber; B) Corneal incision with a 20G angled side port blade; C) Implant mobilization with a saline solution injected with a 27G anterior chamber cannula.
Technique Used to Reposition the Migrated Implant
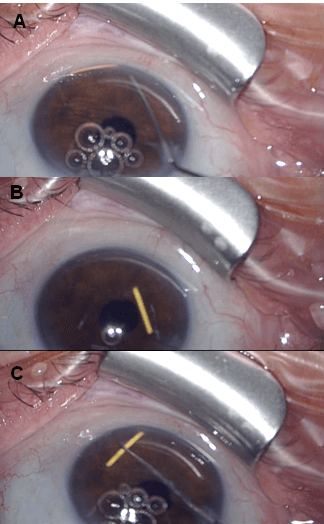
Two days later, the patient was taken to the operating room. Clinical intervention was performed after preparation of the conjunctiva using 5% povidone-iodine solution and under sterile conditions. Under topical anaesthesia, a corneal clear beveled limbal incision into the AC was made with a 20G angled side port blade (Figure 1B) and balanced saline solution was injected with a 27G AC cannula (Figures 1C & 2A), with a successful mobilization of the implant from the inferior angle; at this point, it was floating into the AC (Figure 2B). A reverse sinskey hook was then used (Figures 2C & 3A) to maneuver the ILUVIEN to the posterior cavity between the iris and the IOL successfully (Figures 3B & 3C). Viscoelastic agent was not required and damage to the iris, corneal endothelium and the AC angle was avoided. To prevent re-dislocation of the implant, she was instructed to avoid, to the extent of possible, prone position and any kind of physical effort.
After repositioning of the ILUVIEN implant, the patient was treated with 0.1% dexamethasone/0.3% tobramycin drop therapy in descending dose and, one week after surgery, she reported an improvement in VA. Anterior segment examination showed an improvement of corneal edema, IOP was 16 mmHg and a fundoscopy revealed that the FAc implant was settled in the posterior cavity. One month later, visual acuity remained of 60 EDTRS letters and central macular thickness was 385µm.
Figure 2: A) & B) Note the 27G anterior chamber cannula injecting saline solution and the ILUVIEN implant already floating in the anterior chamber. C) A reverse sinskey hook is used to push the implant between iris and intraocular lens.
Figure 3: A) & B) Note the reverse sinskey hook successfully pushing the implant between the iris and the retropupillar lens into the vitreous cavity. C) Anterior segment examination showing the result of repositioning of the ILUVIEN implant.
Conclusion
ILUVIEN sustained release FAc device is an injectable non-biodegradable intravitreal insert devised for sustained release of FAc for up to 36 months (release rate of 0.2 μg/day). It is a cylindrical polyimide tube, 3.5 mm long and 0,37 mm in diameter loaded with 190 micrograms of fluocinolone acetonide. This FAc implant has marketing authorization for chronic DME treatment after an inadequate response to prior therapy and for prevention of relapse in recurrent non-infectious uveitis affecting the posterior segment. The clinical efficacy of ILUVIEN was demonstrated in the FAME clinical trials [2, 4].
The most frequently reported adverse drug reactions in the FAME studies included increased IOP (38% of DME studies subjects required IOP-lowering medication, 4.8% required IOP-lowering surgeries), cataract (incidence of approximately 82%) and conjunctival haemorrhage. More serious adverse reactions although less frequently reported include optic disc haemorrhage and retinal detachment. The long-term safety repercussions of retention of the non-biodegradable device inside the vitreous cavity are not known although ILUVIEN is made of polyimide which is essentially similar to an IOL haptic and, therefore, it is expected to remain inert inside the eye [2, 5].
Emerging real-life data demonstrates that ILUVIEN has shown to have a predictable side effect profile and although uncommon, this implant can potentially migrate into the AC [4]. There are only 3 reported cases in the literature and all of them in vitrectomized eyes (maybe because the device would not be tethered by vitreous) with posterior capsular tears in relation to previous complicated cataract procedures, sulcus or iris clip lens placement and zonular weakness [3, 6]. In these cases, alternative DME treatments should be considered and if ILUVIEN is elected they should be monitored closely.
Compared to ILUVIEN which is a relatively new tool, Ozurdex (6 mm by 0,46 mm, 0,7 mg Ozurdex; Allergan, Irvine, CA) has been widely used and there is extensive experience when referring to implant migration which is, also, a rare complication [7]. It has been associated to vision-threatening complications that involve permanent corneal decompensation and elevation of the IOP and previous series suggests that surgical removal of the implant is mandatory as they may be severe enough to warrant keratoplasty. Corneal endothelial toxicity has been associated to chemical toxicity as well as mechanical trauma from the rigid implant -the corneal edema was noted if the migration occurred in the early postoperative period and decreased in incidence when it occurred later probably due to the implant decreased rigidity. Other studies suggest that steroid implants placed close to the trabecular meshwork or ciliary body turns out in a higher incidence of raised IOP. Current literature regarding ILUVIEN dislocation does not report severe corneal complications possibly due to prompt repositioning together with the smaller size of the FAc implant compared to the Ozurdex.
Multiple surgical techniques have been described to relocate/remove Ozurdex implant including the use of forceps, Nd:YAG laser to fragment the implant, aspiration of the disintegrated device, relocation with a 30-gauge needle, implant suturing to the sclera to prevent remigration or a no-touch techniques for implant removal using viscoelastic [7-10]. Nonsurgical management with supine positioning after pharmacologic pupillary dilation has, also, been described [11]. Khurana et al. described unsuccessful attempts to grasp the Ozurdex implant with forceps resulting it in disintegration [7].
We report a simple and quick technique for surgical repositioning a fluocinolone acetonide implant that required minimal manipulation and with minimal tissue disturbance. The non-biodegradable nature of ILUVIEN made the manipulation easier without compromising implant integrity and effectiveness. Mild corneal decompensation completely resolved in a week possibly due to prompt repositioning and IOP decreased within statistically normal limits. However, the risk of re-migration into the AC persists as the shell of the implant is nonbiodegradable; the use of miotics and avoiding the prone position could help prevent the recurrence.
In conclusion, we present the fourth case of migration of ILUVIEN into the AC, in our case in the context of a previous iris-claw lens implantation, and a simple technique for ease relocation to the vitreous cavity. It is important to be cautious while using a FAc implant in patients with capsular defects, zonular weakness, and previous vitrectomy; thus, a regular follow-up and early detection of migration may ensure prompt removal which is essential to prevent corneal edema and damage from raised IOP. We recommend using a reverse sinskey hook as a smaller entry incision can be achieved to maintain the sealing of the AC. In conclusion, our technique showed that the implant was relocated into the posterior cavity without compromising the implant integrity and without losing the effectiveness of the drug delivery system.
Abbreviations
DME: Diabetic Macular Edema
FAc: Fluocinolone Acetonide
AC: Anterior Chamber
IOP: Intraocular Pressure
OL: Intraocular Lens
CMT: Central Macular Thickness
VA: Visual Acuity
Conflicts of Interest
None.
Funding
None.
Consent
The authors state that written informed consent for medical information and images to be published was provided by the patient.
Financial Interest
None.
Article Info
Article Type
Case ReportPublication history
Received: Wed 19, Aug 2020Accepted: Mon 31, Aug 2020
Published: Mon 07, Sep 2020
Copyright
© 2023 Clara Monferrer Adsuara. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.SCR.2020.09.05
Author Info
Clara Monferrer Adsuara Lucía Mata-Moret Verónica Castro-Navarro Javier Montero-Hernández
Corresponding Author
Clara Monferrer AdsuaraDepartment of Ophthalmology, Consorcio Hospital General Universitario of Valencia, Valencia, Spain
Figures & Tables

References
- Georgios D Panos (2020) Advances in intravitreal therapy and implants: where are we now? Ther Deliv 11: 69-73. [Crossref]
- ILUVIEN 190 micrograms intravitreal implant in applicator. 2020.
- Ibraheem A El Ghrably, Ahmed Saad, Christiana Dinah (2015) A Novel Technique for Repositioning of a Migrated ILUVIEN(®) (Fluocinolone Acetonide) Implant into the Anterior Chamber. Ophthalmol Ther 4: 129-133. [Crossref]
- Sarah E Holden, Barry Kapik, Annette B Beiderbeck, Craig J Currie (2019) Comparison of data characterizing the clinical effectiveness of the fluocinolone intravitreal implant (ILUVIEN) in patients with diabetic macular edema from the real world, non-interventional ICE-UK study and the FAME randomized controlled trials. Curr Med Res Opin 35: 1165-1176. [Crossref]
- Dilraj S Grewal, Donald C Fletcher, Seenu M Hariprasad, Ivan J Suner (2019) Effect of fluocinolone acetonide 0.2 mug/day implant on the decision to drive in patients with diabetic macular oedema: a report from the FAME study. BMJ Open Ophthalmol 4: e000405. [Crossref]
- Vasileios T Papastavrou, Hadi Zambarakji, Ian Dooley, Haralabos Eleftheriadis, Timothy L Jackson (2017) Observation: Fluocinolone Acetonide (ILUVIEN) implant migration into the anterior chamber. Retin Cases Brief Rep 11: 44-46. [Crossref]
- Rahul N Khurana, Suri N Appa, Colin A McCannel, Michael J Elman, Susan E Wittenberg et al. (2014) Dexamethasone implant anterior chamber migration: risk factors, complications, and management strategies. Ophthalmology 121: 67-71. [Crossref]
- José I Vela, Jaume Crespí, David Andreu (2012) Repositioning of dexamethasone intravitreal implant (Ozurdex) migrated into the anterior chamber. Int Ophthalmol 32: 583-584. [Crossref]
- Carlos Mateo, Micol Alkabes, Anniken Burés Jelstrup (2014) Scleral fixation of dexamethasone intravitreal implant (OZURDEX) in a case of angle-supported lens implantation. Int Ophthalmol 34: 661-665. [Crossref]
- Jay M Stewart (2020) Modified No-Touch Technique for Removal of a Dexamethasone Implant that has Migrated to the Anterior Chamber. Ocul Immunol Inflamm 28: 238-9. [Crossref]
- Priya Srinivasan, Chaitra Jayadev, Rohit Shetty (2017) The nomadic Ozurdex: Anterior migration of the dexamethasone implant and back! Oman J Ophthalmol 10: 109-111. [Crossref]
